IL-23 promotes TCR-mediated negative selection of thymocytes through the upregulation of IL-23 receptor and RORγt
- PMID: 25001511
- PMCID: PMC4136447
- DOI: 10.1038/ncomms5259
IL-23 promotes TCR-mediated negative selection of thymocytes through the upregulation of IL-23 receptor and RORγt
Abstract
Transient thymic involution is frequently found during inflammation, yet the mode of action of inflammatory cytokines is not well defined. Here we report that interleukin-23 (IL-23) production by the thymic dendritic cells (DCs) promotes apoptosis of the CD4(hi)CD8(hi) double-positive (DP) thymocytes. A deficiency in IL-23 signalling interferes with negative selection in the male D(b)/H-Y T-cell receptor (TCR) transgenic mice. IL-23 plus TCR signalling results in significant upregulation of IL-23 receptor (IL-23R) expressed predominantly on CD4(hi)CD8(hi)CD3(+)αβTCR(+) DP thymocytes, and leads to RORγt-dependent apoptosis. These results extend the action of IL-23 beyond its peripheral effects to a unique role in TCR-mediated negative selection including elimination of natural T regulatory cells in the thymus.
Conflict of interest statement
Figures




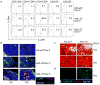
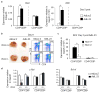

Similar articles
-
Plasma membrane sphingomyelin modulates thymocyte development by inhibiting TCR-induced apoptosis.Int Immunol. 2019 Mar 28;31(4):211-223. doi: 10.1093/intimm/dxy082. Int Immunol. 2019. PMID: 30561621
-
Interleukin-22 drives endogenous thymic regeneration in mice.Science. 2012 Apr 6;336(6077):91-5. doi: 10.1126/science.1218004. Epub 2012 Mar 1. Science. 2012. PMID: 22383805 Free PMC article.
-
The T cell receptor repertoire of CD4-8+ thymocytes is altered by overexpression of the BCL-2 protooncogene in the thymus.J Exp Med. 1994 Jan 1;179(1):145-53. doi: 10.1084/jem.179.1.145. J Exp Med. 1994. PMID: 8270861 Free PMC article.
-
Batf-dependent Th17 cells critically regulate IL-23 driven colitis-associated colon cancer.Gut. 2016 Jul;65(7):1139-50. doi: 10.1136/gutjnl-2014-308227. Epub 2015 Apr 2. Gut. 2016. PMID: 25838550
-
Regulation of Negative Selection in the Thymus by Cytokines: Novel Role of IL-23 to Regulate RORγt.In: Soboloff J, Kappes DJ, editors. Signaling Mechanisms Regulating T Cell Diversity and Function. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 3. In: Soboloff J, Kappes DJ, editors. Signaling Mechanisms Regulating T Cell Diversity and Function. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 3. PMID: 30427634 Free Books & Documents. Review.
Cited by
-
The IL-23R and Its Genetic Variants: A Hitherto Unforeseen Bridge Between the Immune System and Cancer Development.Cancers (Basel). 2024 Dec 27;17(1):55. doi: 10.3390/cancers17010055. Cancers (Basel). 2024. PMID: 39796684 Free PMC article. Review.
-
Thymflammation: The Role of a Constitutively Active Inflammatory Network and "Ectopic" Cell Types in the Thymus in the Induction of T Cell Tolerance and Beyond.Immunol Rev. 2025 Jul;332(1):e70037. doi: 10.1111/imr.70037. Immunol Rev. 2025. PMID: 40433806 Free PMC article. Review.
-
IL-23 Promotes a Coordinated B Cell Germinal Center Program for Class-Switch Recombination to IgG2b in BXD2 Mice.J Immunol. 2020 Jul 15;205(2):346-358. doi: 10.4049/jimmunol.2000280. Epub 2020 Jun 17. J Immunol. 2020. PMID: 32554431 Free PMC article.
-
Interleukin-23 deficiency alters thymic selection in lupus-prone mice.Lupus. 2019 Jul;28(8):1007-1012. doi: 10.1177/0961203319854804. Epub 2019 Jun 5. Lupus. 2019. PMID: 31166866 Free PMC article.
-
CD4+ CCR6+ T cells, but not γδ T cells, are important for the IL-23R-dependent progression of antigen-induced inflammatory arthritis in mice.Eur J Immunol. 2020 Feb;50(2):245-255. doi: 10.1002/eji.201948112. Epub 2019 Nov 28. Eur J Immunol. 2020. PMID: 31778214 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI027767/AI/NIAID NIH HHS/United States
- 3R01AI71110-02S1/AI/NIAID NIH HHS/United States
- P30 AR046031/AR/NIAMS NIH HHS/United States
- I01 BX000600/BX/BLRD VA/United States
- 1R01 AI083705/AI/NIAID NIH HHS/United States
- P30 AR46031/AR/NIAMS NIH HHS/United States
- R01 AI083705/AI/NIAID NIH HHS/United States
- I01 BX004049/BX/BLRD VA/United States
- R01 AI071110/AI/NIAID NIH HHS/United States
- P30 DK079626/DK/NIDDK NIH HHS/United States
- P30 CA013148/CA/NCI NIH HHS/United States
- 1AI 071110/AI/NIAID NIH HHS/United States
- P30 AR048311/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

