Brucella melitensis invades murine erythrocytes during infection
- PMID: 25001604
- PMCID: PMC4187840
- DOI: 10.1128/IAI.01779-14
Brucella melitensis invades murine erythrocytes during infection
Abstract
Brucella spp. are facultative intracellular Gram-negative coccobacilli responsible for brucellosis, a worldwide zoonosis. We observed that Brucella melitensis is able to persist for several weeks in the blood of intraperitoneally infected mice and that transferred blood at any time point tested is able to induce infection in naive recipient mice. Bacterial persistence in the blood is dramatically impaired by specific antibodies induced following Brucella vaccination. In contrast to Bartonella, the type IV secretion system and flagellar expression are not critically required for the persistence of Brucella in blood. ImageStream analysis of blood cells showed that following a brief extracellular phase, Brucella is associated mainly with the erythrocytes. Examination by confocal microscopy and transmission electron microscopy formally demonstrated that B. melitensis is able to invade erythrocytes in vivo. The bacteria do not seem to multiply in erythrocytes and are found free in the cytoplasm. Our results open up new areas for investigation and should serve in the development of novel strategies for the treatment or prophylaxis of brucellosis. Invasion of erythrocytes could potentially protect the bacterial cells from the host's immune response and hamper antibiotic treatment and suggests possible Brucella transmission by bloodsucking insects in nature.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




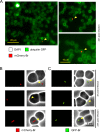
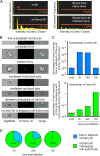
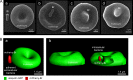
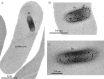
References
-
- Godfroid J, Cloeckaert A, Liautard JP, Kohler S, Fretin D, Walravens K, Garin-Bastuji B, Letesson JJ. 2005. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 36:313–326. 10.1051/vetres:2005003 - DOI - PubMed
-
- Zheludkov MM, Tsirel'son LE. 2010. Reservoirs of Brucella infection in nature. Biol. Bull. 37:709–715. 10.1134/S106235901007006X - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

