Effect of nonheme iron-containing ferritin Dpr in the stress response and virulence of pneumococci
- PMID: 25001605
- PMCID: PMC4187797
- DOI: 10.1128/IAI.01829-14
Effect of nonheme iron-containing ferritin Dpr in the stress response and virulence of pneumococci
Abstract
Streptococcus pneumoniae (pneumococcus) produces hydrogen peroxide as a by-product of metabolism and provides a competitive advantage against cocolonizing bacteria. As pneumococci do not produce catalase or an inducible regulator of hydrogen peroxide, the mechanism of resistance to hydrogen peroxide is unclear. A gene responsible for resistance to hydrogen peroxide and iron in other streptococci is that encoding nonheme iron-containing ferritin, dpr, but previous attempts to study this gene in pneumococcus by generating a dpr mutant were unsuccessful. In the current study, we found that dpr is in an operon with the downstream genes dhfr and clpX. We generated a dpr deletion mutant which displayed normal early-log-phase and mid-log-phase growth in bacteriologic medium but survived less well at stationary phase; the addition of catalase partially rescued the growth defect. We showed that the dpr mutant is significantly more sensitive to pH, heat, iron concentration, and oxidative stress due to hydrogen peroxide. Using a mouse model of colonization, we also showed that the dpr mutant displays a reduced ability to colonize and is more rapidly cleared from the nasopharynx. Our results thus suggest that Dpr is important for pneumococcal resistance to stress and for nasopharyngeal colonization.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
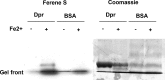



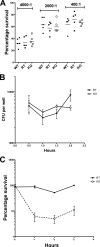

Similar articles
-
Interplay between manganese and iron in pneumococcal pathogenesis: role of the orphan response regulator RitR.Infect Immun. 2013 Feb;81(2):421-9. doi: 10.1128/IAI.00805-12. Epub 2012 Nov 26. Infect Immun. 2013. PMID: 23184523 Free PMC article.
-
An iron-binding protein, Dpr, decreases hydrogen peroxide stress and protects Streptococcus pyogenes against multiple stresses.Infect Immun. 2008 Sep;76(9):4038-45. doi: 10.1128/IAI.00477-08. Epub 2008 Jun 9. Infect Immun. 2008. PMID: 18541662 Free PMC article.
-
Disruption of a Novel Iron Transport System Reverses Oxidative Stress Phenotypes of a dpr Mutant Strain of Streptococcus mutans.J Bacteriol. 2018 Jun 25;200(14):e00062-18. doi: 10.1128/JB.00062-18. Print 2018 Jul 15. J Bacteriol. 2018. PMID: 29735760 Free PMC article.
-
Dual Role of Hydrogen Peroxide as an Oxidant in Pneumococcal Pneumonia.Antioxid Redox Signal. 2021 Apr 20;34(12):962-978. doi: 10.1089/ars.2019.7964. Epub 2020 Aug 14. Antioxid Redox Signal. 2021. PMID: 32283950 Free PMC article. Review.
-
Streptococcus pneumoniae two-component regulatory systems: The interplay of the pneumococcus with its environment.Int J Med Microbiol. 2018 Aug;308(6):722-737. doi: 10.1016/j.ijmm.2017.11.012. Epub 2017 Nov 26. Int J Med Microbiol. 2018. PMID: 29221986 Review.
Cited by
-
Identification and Tetramer Structure of Hemin-Binding Protein SPD_0310 Linked to Iron Homeostasis and Virulence of Streptococcus pneumoniae.mSystems. 2022 Jun 28;7(3):e0022122. doi: 10.1128/msystems.00221-22. Epub 2022 Apr 13. mSystems. 2022. PMID: 35414267 Free PMC article.
-
Isolation and Characterization of Human Monoclonal Antibodies to Pneumococcal Capsular Polysaccharide 3.Microbiol Spectr. 2021 Dec 22;9(3):e0144621. doi: 10.1128/Spectrum.01446-21. Epub 2021 Nov 10. Microbiol Spectr. 2021. PMID: 34756090 Free PMC article.
-
A Capsular Polysaccharide-Specific Antibody Alters Streptococcus pneumoniae Gene Expression during Nasopharyngeal Colonization of Mice.Infect Immun. 2018 Jun 21;86(7):e00300-18. doi: 10.1128/IAI.00300-18. Print 2018 Jul. Infect Immun. 2018. PMID: 29735523 Free PMC article.
-
Electronic Cigarette (E-Cigarette) Vapor Exposure Alters the Streptococcus pneumoniae Transcriptome in a Nicotine-Dependent Manner without Affecting Pneumococcal Virulence.Appl Environ Microbiol. 2020 Jan 21;86(3):e02125-19. doi: 10.1128/AEM.02125-19. Print 2020 Jan 21. Appl Environ Microbiol. 2020. PMID: 31791951 Free PMC article.
-
A ferritin-like protein with antioxidant activity in Ureaplasma urealyticum.BMC Microbiol. 2015 Jul 26;15:145. doi: 10.1186/s12866-015-0485-6. BMC Microbiol. 2015. PMID: 26209240 Free PMC article.
References
-
- Pericone CD, Park S, Imlay JA, Weiser JN. 2003. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J. Bacteriol. 185:6815–6825. 10.1128/JB.185.23.6815-6825.2003 - DOI - PMC - PubMed
-
- Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, Heidelberg J, DeBoy RT, Haft DH, Dodson RJ, Durkin AS, Gwinn M, Kolonay JF, Nelson WC, Peterson JD, Umayam LA, White O, Salzberg SL, Lewis MR, Radune D, Holtzapple E, Khouri H, Wolf AM, Utterback TR, Hansen CL, McDonald LA, Feldblyum TV, Angiuoli S, Dickinson T, Hickey EK, Holt IE, Loftus BJ, Yang F, Smith HO, Venter JC, Dougherty BA, Morrison DA, Hollingshead SK, Fraser CM. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498–506. 10.1126/science.1061217 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

