Retinoid levels influence enterohemorrhagic Escherichia coli infection and Shiga toxin 2 susceptibility in mice
- PMID: 25001607
- PMCID: PMC4187814
- DOI: 10.1128/IAI.02191-14
Retinoid levels influence enterohemorrhagic Escherichia coli infection and Shiga toxin 2 susceptibility in mice
Abstract
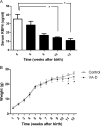
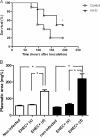
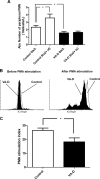
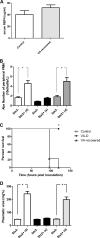
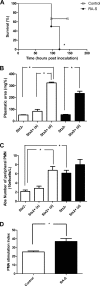
Enterohemorrhagic Escherichia coli (EHEC) is a food-borne pathogen that produces Shiga toxin (Stx) and causes hemorrhagic colitis. Under some circumstances, Stx produced within the intestinal tract enters the bloodstream, leading to systemic complications that may cause the potentially fatal hemolytic-uremic syndrome. Although retinoids like vitamin A (VA) and retinoic acid (RA) are beneficial to gut integrity and the immune system, the effect of VA supplementation on gastrointestinal infections of different etiologies has been controversial. Thus, the aim of this work was to study the influence of different VA status on the outcome of an EHEC intestinal infection in mice. We report that VA deficiency worsened the intestinal damage during EHEC infection but simultaneously improved survival. Since death is associated mainly with Stx toxicity, Stx was intravenously inoculated to analyze whether retinoid levels affect Stx susceptibility. Interestingly, while VA-deficient (VA-D) mice were resistant to a lethal dose of Stx2, RA-supplemented mice were more susceptible to it. Given that peripheral blood polymorphonuclear cells (PMNs) are known to potentiate Stx2 toxicity, we studied the influence of retinoid levels on the absolute number and function of PMNs. We found that VA-D mice had decreased PMN numbers and a diminished capacity to produce reactive oxygen species, while RA supplementation had the opposite effect. These results are in line with the well-known function of retinoids in maintaining the homeostasis of the gut but support the idea that they have a proinflammatory effect by acting, in part, on the PMN population.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Promoter sequence of Shiga toxin 2 (Stx2) is recognized in vivo, leading to production of biologically active Stx2.mBio. 2013 Oct 1;4(5):e00501-13. doi: 10.1128/mBio.00501-13. mBio. 2013. PMID: 24085779 Free PMC article.
-
Prophage induction, but not production of phage particles, is required for lethal disease in a microbiome-replete murine model of enterohemorrhagic E. coli infection.PLoS Pathog. 2019 Jan 10;15(1):e1007494. doi: 10.1371/journal.ppat.1007494. eCollection 2019 Jan. PLoS Pathog. 2019. PMID: 30629725 Free PMC article.
-
Leukotriene C4 increases the susceptibility of adult mice to Shiga toxin-producing Escherichia coli infection.Int J Med Microbiol. 2015 Dec;305(8):910-7. doi: 10.1016/j.ijmm.2015.09.006. Epub 2015 Sep 16. Int J Med Microbiol. 2015. PMID: 26456732
-
Citrobacter rodentium(ϕStx2dact), a murine infection model for enterohemorrhagic Escherichia coli.Curr Opin Microbiol. 2022 Feb;65:183-190. doi: 10.1016/j.mib.2021.11.013. Epub 2021 Dec 17. Curr Opin Microbiol. 2022. PMID: 34929548 Free PMC article. Review.
-
Shiga toxin interaction with human intestinal epithelium.Toxins (Basel). 2011 Jun;3(6):626-39. doi: 10.3390/toxins3060626. Epub 2011 Jun 14. Toxins (Basel). 2011. PMID: 22069729 Free PMC article. Review.
Cited by
-
High-fat and high-carbohydrate diets worsen the mouse brain susceptibility to damage produced by enterohemorrhagic Escherichia coli Shiga toxin 2.Heliyon. 2024 Oct 26;10(21):e39871. doi: 10.1016/j.heliyon.2024.e39871. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39553573 Free PMC article.
-
Modulation of Intestinal Immune and Barrier Functions by Vitamin A: Implications for Current Understanding of Malnutrition and Enteric Infections in Children.Nutrients. 2018 Aug 21;10(9):1128. doi: 10.3390/nu10091128. Nutrients. 2018. PMID: 30134532 Free PMC article. Review.
-
Commensal segmented filamentous bacteria-derived retinoic acid primes host defense to intestinal infection.Cell Host Microbe. 2021 Dec 8;29(12):1744-1756.e5. doi: 10.1016/j.chom.2021.09.010. Epub 2021 Oct 21. Cell Host Microbe. 2021. PMID: 34678170 Free PMC article.
-
Differential Outcome between BALB/c and C57BL/6 Mice after Escherichia coli O157:H7 Infection Is Associated with a Dissimilar Tolerance Mechanism.Infect Immun. 2021 Apr 16;89(5):e00031-21. doi: 10.1128/IAI.00031-21. Print 2021 Apr 16. Infect Immun. 2021. PMID: 33619029 Free PMC article.
-
Vitamin A at the interface of host-commensal-pathogen interactions.PLoS Pathog. 2019 Jun 6;15(6):e1007750. doi: 10.1371/journal.ppat.1007750. eCollection 2019 Jun. PLoS Pathog. 2019. PMID: 31170262 Free PMC article. Review. No abstract available.
References
-
- Rivas M, Miliwebsky E, Chinen I, Roldan CD, Balbi L, Garcia B, Fiorilli G, Sosa-Estani S, Kincaid J, Rangel J, Griffin PM. 2006. Characterization and epidemiologic subtyping of Shiga toxin-producing Escherichia coli strains isolated from hemolytic uremic syndrome and diarrhea cases in Argentina. Foodborne Pathog. Dis. 3:88–96. 10.1089/fpd.2006.3.88 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

