Clinical outcomes associated with proton pump inhibitor use among clopidogrel-treated patients within CYP2C19 genotype groups following acute myocardial infarction
- PMID: 25001880
- PMCID: PMC4287459
- DOI: 10.1038/tpj.2014.28
Clinical outcomes associated with proton pump inhibitor use among clopidogrel-treated patients within CYP2C19 genotype groups following acute myocardial infarction
Abstract
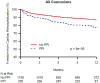
We examined clinical outcomes with proton pump inhibitors (PPI) use within CYP2C19 genotype groups during clopidogrel treatment following acute myocardial infarction (AMI). 2062 patients were genotyped for CYP2C19*2 and *17 variants in TRIUMPH. 12 month clinical outcomes were analyzed among patients discharged on clopidogrel within CYP2C19*2 carrier, CYP2C19*17 carrier, and CYP2C19*1 homozygote genotype groups. PPI use was not associated with a difference in mortality. Among clopidogrel-treated Caucasians following AMI, PPI use was associated with a significantly higher rate of cardiac rehospitalization (HR 1.62, 95% CI 1.19-2.19; P=0.002) compared with no PPI use. PPI users who were carriers of the CYP2C19*17 variant experienced significantly higher rates of cardiac rehospitalization (HR 2.05, 95% CI 1.26-3.33; P=0.003), carriers of the CYP2C19*2 variant had a trend toward increased 1-year cardiac rehospitalization (HR 1.69, 95% CI 0.95-2.99; P=0.07), while no significant differences were observed among CYP2C19*1 homozygotes. These results indicate that the risks associated with PPI use among clopidogrel-treated Caucasian post-MI patients are impacted by CYP2C19 genotype, and suggest knowledge of genotype may be useful for personalizing PPI use among patients following AMI to reduce rehospitalization.
Conflict of interest statement
Petra A. Lenzini: None
David E. Lanfear: None
Tracy Y. Wang: Research Grants: Lilly USA (Significant), Daiichi Sankyo (Significant), and Gilead Science (Significant); Consultant: Astra Zeneca (Modest), American College of Cardiology Foundation (Significant)
John A. Spertus: Research Grants: Eli Lilly, EveHeart, Genentech, Gilead; Consultant: St. Jude Medical (modest), United Healthcare (modest), Amgen (modest), Gilead (modest), Genentech (modest), Janssen (modest), Novartis (modest); Copyrights/Patents: Seattle Angina Questionnaire, Kansas City Cardiomyopathy Questionnaire, Peripheral Artery Questionnaire, US Patents: 7,643,969; 7,853,456; 12/965,656; 13/615,401
Richard G. Bach: Research Grants: AstraZeneca, Eli Lilly, Bristol-Myers Squibb, Merck/Schering-Plough; Consultant (CEC Activity only): Roche (Significant), Pfizer (Modest)
Sharon Cresci: None
Figures



References
-
- Bhatt DL. Role of antiplatelet therapy across the spectrum of patients with coronary artery disease. Am J Cardiol. 2009;103:11A–19A. - PubMed
-
- Depta JP, Bhatt DL. Aspirin and platelet adenosine diphosphate receptor antagonists in acute coronary syndromes and percutaneous coronary intervention: role in therapy and strategies to overcome resistance. Am J Cardiovasc Drugs. 2008;8:91–112. - PubMed
-
- Somma KA, Bhatt DL, Fonarow GC, Cannon CP, Cox M, Laskey W, et al. Guideline adherence after ST-segment elevation versus non-ST segment elevation myocardial infarction. Am J Cardiovasc Drugs. 2012;5:654–661. - PubMed
-
- Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

