A double-blind, placebo-controlled intervention trial of 3 and 10 mg sublingual melatonin for post-concussion syndrome in youths (PLAYGAME): study protocol for a randomized controlled trial
- PMID: 25001947
- PMCID: PMC4227124
- DOI: 10.1186/1745-6215-15-271
A double-blind, placebo-controlled intervention trial of 3 and 10 mg sublingual melatonin for post-concussion syndrome in youths (PLAYGAME): study protocol for a randomized controlled trial
Abstract
Background: By the age of sixteen, one in five children will sustain a mild traumatic brain injury also known as concussion. Our research found that one in seven school children with mild traumatic brain injury suffer post-concussion syndrome symptoms for three months or longer. Post-concussion syndrome is associated with significant disability in the child and his/her family and yet there are no evidence-based medical treatments available. Melatonin has several potential mechanisms of action that could be useful following mild traumatic brain injury, including neuroprotective effects. The aim of this study is to determine if treatment with melatonin improves post-concussion syndrome in youths following mild traumatic brain injury. Our hypothesis is that treatment of post-concussion syndrome following mild traumatic brain injury with 3 or 10 mg of sublingual melatonin for 28 days will result in a decrease in post-concussion syndrome symptoms compared with placebo.
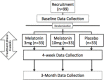
Methods/design: Ninety-nine youths with mild traumatic brain injury, aged between 13 and 18 years, who are symptomatic at 30 days post-injury will be recruited. This study will be conducted as a randomized, double blind, placebo-controlled superiority trial of melatonin. Three parallel treatment groups will be examined with a 1:1:1 allocation: sublingual melatonin 3 mg, sublingual melatonin 10 mg, and sublingual placebo. Participants will receive treatment for 28 days. The primary outcome is a change on the Post-Concussion Symptom Inventory (Parent and Youth). The secondary outcomes will include neurobehavioral function, health-related quality of life and sleep. Neurophysiological and structural markers of change, using magnetic resonance imaging techniques and transcranial magnetic stimulation, will also be investigated.
Discussion: Melatonin is a safe and well-tolerated agent that has many biological properties that may be useful following a traumatic brain injury. This study will determine whether it is a useful treatment for children with post-concussion syndrome. Recruitment commenced on 4 December 2014.
Trial registration: This trial was registered on 6 June 2013 at ClinicalTrials.gov.
Registration number: NCT01874847.
Figures
References
-
- McKinlay A, Grace RC, Horwood LJ, Fergusson DM, Ridder EM, MacFarlane MR. Prevalence of traumatic brain injury among children, adolescents and young adults: prospective evidence from a birth cohort. Brain Inj. 2008;22:175–181. - PubMed
-
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20:229. - PubMed
-
- Maas AIR, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. - PubMed
-
- Gagnon I, Swaine B, Friedman D, Forget R. Exploring children’s self-efficacy related to physical activity performance after a mild traumatic brain injury. J Head Trauma Rehabil. 2005;20:436–449. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

