Role of sphingosine kinase 1 and sphingosine-1-phosphate in CD40 signaling and IgE class switching
- PMID: 25002116
- PMCID: PMC4202100
- DOI: 10.1096/fj.14-251611
Role of sphingosine kinase 1 and sphingosine-1-phosphate in CD40 signaling and IgE class switching
Abstract
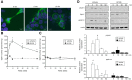
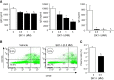
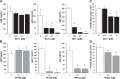
The tumor necrosis factor (TNF) receptor family member CD40 plays an essential role in the activation of antigen-presenting cells, B cell maturation, and immunoglobulin (Ig) class switching critical for adaptive immunity. Although the bioactive sphingolipid metabolite sphingosine-1-phosphate (S1P) and the kinase that produces it, sphingosine kinase 1 (SphK1), have long been implicated in the actions of TNF mediated by engagement of TNFR1, nothing is yet known of their role in CD40-mediated events. We have now found that ligation of CD40 activates and translocates SphK1 to the plasma membrane, leading to generation of S1P. SphK1 inhibition in human tonsil B cells, as well as inhibition or deletion of SphK1 in mouse splenic B cells, significantly reduced CD40-mediated Ig class switching and plasma cell differentiation ex vivo. Optimal activation of downstream CD40 signaling pathways, including NF-κB, p38, and JNK, also required SphK1. In mice treated with a SphK1 inhibitor or in SphK1(-/-) mice, isotype switching to antigen-specific IgE was decreased in vivo by 70 and 55%, respectively. Our results indicate that SphK1 is important for CD40-mediated B cell activation and regulation of humoral responses and suggest that targeting SphK1 might be a useful therapeutic approach to control antigen-specific IgE production.
Keywords: B cells; NF-κB; inflammation; sphingolipids.
© FASEB.
Figures




References
-
- Van Kooten C., Banchereau J. (2000) CD40-CD40 ligand. J. Leukoc. Biol. 67, 2–17 - PubMed
-
- Bishop G. A. (2004) The multifaceted roles of TRAFs in the regulation of B-cell function. Nat. Rev. Immunol. 4, 775–786 - PubMed
-
- Kawabe T., Naka T., Yoshida K., Tanaka T., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T., Kikutani H. (1994) The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 1, 167–178 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30NS047463/NS/NINDS NIH HHS/United States
- P30 NS047463/NS/NINDS NIH HHS/United States
- R01 AI050094/AI/NIAID NIH HHS/United States
- P30 CA016059/CA/NCI NIH HHS/United States
- R25 GM090084/GM/NIGMS NIH HHS/United States
- T32 HL094290/HL/NHLBI NIH HHS/United States
- R37GM043880/GM/NIGMS NIH HHS/United States
- T32HL094290/HL/NHLBI NIH HHS/United States
- U19 AI077435/AI/NIAID NIH HHS/United States
- R01 GM043880/GM/NIGMS NIH HHS/United States
- P30CA16059/CA/NCI NIH HHS/United States
- R01 CA061774/CA/NCI NIH HHS/United States
- R37 GM043880/GM/NIGMS NIH HHS/United States
- U19AI077435/AI/NIAID NIH HHS/United States
- R01AI50094/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

