In vitro cytotoxicity of Artemisia vulgaris L. essential oil is mediated by a mitochondria-dependent apoptosis in HL-60 leukemic cell line
- PMID: 25002129
- PMCID: PMC4227289
- DOI: 10.1186/1472-6882-14-226
In vitro cytotoxicity of Artemisia vulgaris L. essential oil is mediated by a mitochondria-dependent apoptosis in HL-60 leukemic cell line
Abstract
Background: The essential oil (EO) of Artemisia vulgaris L. has been traditionally used worldwide for treating a large number of diseases. Although major components in A. vulgaris EO have been shown to inhibit growth of different cancer cells, as pure compounds or part of other plants extracted oil, no information is known about its anti-proliferative activities. Therefore, the current investigation has evaluated the toxicity of the plant extracted oil from buds (AVO-b) and leaves (AVO-l) and characterized their growth inhibitory effects on cancer cells.
Methods: AVO-b and AVO-l from A. vulgaris L. were extracted by hydrodistillation, and their effect on the viability of human HL-60 promyelocytic leukemia and various other cancer cell lines was tested using MTT assay. Flow cytometric analysis of apoptosis, DNA fragmentation assay, caspases enzymatic activities and Western blotting were used to determine the apoptotic pathway triggered by their action on HL-60 cells.
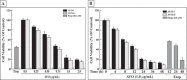
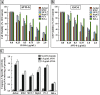
Results: Low concentrations of AVO-b and AVO-l inhibited the growth of HL-60 cells in a dose- and time-dependent manner. Employing flow cytometric, DNA fragmentation and caspase activation analyses, demonstrated that the cytotoxic effect of the oils is mediated by a caspase-dependent apoptosis. Kinetic studies in the presence and absence specific caspase inhibitors showed that activation of caspase-8 was dependent and subsequent to the activation of caspases-9 and -3. In addition, the essential oil caused a disruption of the mitochondrial transmembrane potential (ΔΨm), increased the release of cytochrome c to the cytosol, and altered the expression of certain members of Bcl-2 family (Bcl-2, Bax and Bid), Apaf-1 and XIAP. Interestingly, low doses of AVO-b and AVO-1 also induced apoptosis in various cancer cell lines, but not in noncancerous cells.
Conclusions: The results demonstrate that the EO-induced apoptosis in HL-60 cells is mediated by caspase-dependent pathways, involving caspases-3, -9, and -8, which are initiated by Bcl-2/Bax/Bid-dependent loss of ΔΨm leading to release of cytochrome c to the cytoplasm to activate the caspase cascade. The finding that AVO-b and AVO-l are more efficient to induce apoptosis in different cancer cell lines than noncancerous cells, suggests that A. vulgaris might be a promising source for new anticancer agents.
Figures





References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Klein G. Cancer, apoptosis, and nonimmune surveillance. Cell Death Differ. 2004;11(1):13–17. - PubMed
-
- Hu W, Kavanagh JJ. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003;4(12):721–729. - PubMed
-
- Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256(1):42–49. - PubMed
-
- Alnemri ES. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem. 1997;64(1):33–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

