Traditional Chinese medicine (Shun-Qi-Tong-Xie Granule) for irritable bowel syndrome: study protocol for a randomised controlled trial
- PMID: 25002196
- PMCID: PMC4104736
- DOI: 10.1186/1745-6215-15-273
Traditional Chinese medicine (Shun-Qi-Tong-Xie Granule) for irritable bowel syndrome: study protocol for a randomised controlled trial
Abstract
Background: Irritable bowel syndrome (IBS) is a common gastrointestinal functional disorder with no effective therapy. Traditional Chinese medicine (TCM) is one of the most common complementary therapies in China. We designed this study to evaluate the efficacy and safety of Shun-Qi-Tong-Xie Granule (SQTX Granule), a TCM treatment, in patients with IBS with diarrhea (IBS-D).
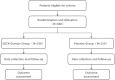
Methods/design: A randomised, double-blinded, placebo-controlled, multi-centre, superiority clinical trial to evaluate the efficacy and safety of SQTX Granule is proposed. Eligible patients (Rome III) with IBD-S will be randomly assigned into SQTX Granule group and the placebo group. Patients will receive a 28-day treatment and a 2-month follow-up. The primary outcome measures include the scores of IBS-quality of life (IBS-QOL) rating scale and IBS-symptom severity scale (IBS-SSS) rating scale. The secondary outcome measures include the improvement of symptom scores, and the duration of abdominal pain and diarrhea.
Discussion: According to TCM theory, SQTX Granule has a regulating effect on abdominal pain, diarrhea and the syndrome of liver-spleen disharmony, which is similar to the symptoms of IBS-D. This study will provide objective evidence to evaluate the efficiency and safety of SQTX Granule in IBS-D treatment.
Trial registration: ChiCTR-TRC-14004241. Date of registration: 9 February 2014.
Figures


References
-
- Agréus L, Svärdsudd K, Nyrén O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109:671–680. - PubMed
-
- Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(Supp 1):S1–S35. - PubMed
-
- Rey E, Talley NJ. Irritable bowel syndrome: novel views on the epidemiology and potential risk factors. Dig Liver Dis. 2009;41:772–780. - PubMed
-
- Talley NJ, Zinsmeister AR, Melton LJ 3rd. Irritable bowel syndrome in a community: symptom subgroups, risk factors, and health care utilization. Am J Epidemiol. 1995;142:76–83. - PubMed
-
- Thompson WG, Irvine EJ, Pare P, Ferrazzi S, Rance L. Functional gastrointestinal disorders in Canada: first population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci. 2002;47:225–235. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

