Insights into the gating mechanism of the ryanodine-modified human cardiac Ca2+-release channel (ryanodine receptor 2)
- PMID: 25002270
- PMCID: PMC4216943
- DOI: 10.1124/mol.114.093757
Insights into the gating mechanism of the ryanodine-modified human cardiac Ca2+-release channel (ryanodine receptor 2)
Abstract
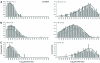
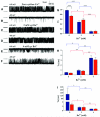
Ryanodine receptors (RyRs) are intracellular membrane channels playing key roles in many Ca(2+) signaling pathways and, as such, are emerging novel therapeutic and insecticidal targets. RyRs are so named because they bind the plant alkaloid ryanodine with high affinity and although it is established that ryanodine produces profound changes in all aspects of function, our understanding of the mechanisms underlying altered gating is minimal. We address this issue using detailed single-channel gating analysis, mathematical modeling, and energetic evaluation of state transitions establishing that, with ryanodine bound, the RyR pore adopts an extremely stable open conformation. We demonstrate that stability of this state is influenced by interaction of divalent cations with both activating and inhibitory cytosolic sites and, in the absence of activating Ca(2+), trans-membrane voltage. Comparison of the conformational stability of ryanodine- and Imperatoxin A-modified channels identifies significant differences in the mechanisms of action of these qualitatively similar ligands.
Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics.
Figures






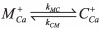


Similar articles
-
Peptide probe of ryanodine receptor function. Imperatoxin A, a peptide from the venom of the scorpion Pandinus imperator, selectively activates skeletal-type ryanodine receptor isoforms.J Biol Chem. 1995 Dec 1;270(48):28696-704. doi: 10.1074/jbc.270.48.28696. J Biol Chem. 1995. PMID: 7499390
-
A mechanistic description of gating of the human cardiac ryanodine receptor in a regulated minimal environment.J Gen Physiol. 2012 Aug;140(2):139-58. doi: 10.1085/jgp.201110706. Epub 2012 Jul 16. J Gen Physiol. 2012. PMID: 22802361 Free PMC article.
-
Light at the end of the Ca(2+)-release channel tunnel: structures and mechanisms involved in ion translocation in ryanodine receptor channels.Q Rev Biophys. 2001 Feb;34(1):61-104. doi: 10.1017/s0033583501003675. Q Rev Biophys. 2001. PMID: 11388090 Review.
-
Ryanoids and imperatoxin affect the modulation of cardiac ryanodine receptors by dihydropyridine receptor Peptide A.Biochim Biophys Acta. 2008 Nov;1778(11):2469-79. doi: 10.1016/j.bbamem.2008.07.024. Epub 2008 Aug 3. Biochim Biophys Acta. 2008. PMID: 18722342 Free PMC article.
-
Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites.Clin Exp Pharmacol Physiol. 2007 Sep;34(9):889-96. doi: 10.1111/j.1440-1681.2007.04708.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17645636 Review.
Cited by
-
Differential Protein Expression in Congenital and Acquired Cholesteatomas.PLoS One. 2015 Sep 3;10(9):e0137011. doi: 10.1371/journal.pone.0137011. eCollection 2015. PLoS One. 2015. PMID: 26335306 Free PMC article.
-
Pulsed infrared releases Ca2+ from the endoplasmic reticulum of cultured spiral ganglion neurons.J Neurophysiol. 2018 Aug 1;120(2):509-524. doi: 10.1152/jn.00740.2017. Epub 2018 Apr 18. J Neurophysiol. 2018. PMID: 29668377 Free PMC article.
-
Unambiguous observation of blocked states reveals altered, blocker-induced, cardiac ryanodine receptor gating.Sci Rep. 2016 Oct 5;6:34452. doi: 10.1038/srep34452. Sci Rep. 2016. PMID: 27703263 Free PMC article.
-
Isolated Cardiac Ryanodine Receptor Function Varies Between Mammals.J Membr Biol. 2024 Apr;257(1-2):25-36. doi: 10.1007/s00232-023-00301-0. Epub 2024 Jan 29. J Membr Biol. 2024. PMID: 38285125 Free PMC article.
References
-
- Amador FJ, Stathopulos PB, Enomoto M, Ikura M. Ryanodine receptor calcium release channels: lessons from structure-function studies. FEBS J. 2013;280:5456–5470. - PubMed
-
- Armisén R, Sierralta J, Vélez P, Naranjo D, Suárez-Isla BA. Modal gating in neuronal and skeletal muscle ryanodine-sensitive Ca2+ release channels. Am J Physiol. 1996;271:C144–C153. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Bezanilla F. How membrane proteins sense voltage. Nat Rev Mol Cell Biol. 2008;9:323–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

