The novel α7β2-nicotinic acetylcholine receptor subtype is expressed in mouse and human basal forebrain: biochemical and pharmacological characterization
- PMID: 25002271
- PMCID: PMC4152907
- DOI: 10.1124/mol.114.093377
The novel α7β2-nicotinic acetylcholine receptor subtype is expressed in mouse and human basal forebrain: biochemical and pharmacological characterization
Abstract
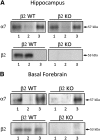
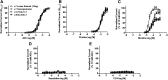
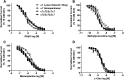
We examined α7β2-nicotinic acetylcholine receptor (α7β2-nAChR) expression in mammalian brain and compared pharmacological profiles of homomeric α7-nAChRs and α7β2-nAChRs. α-Bungarotoxin affinity purification or immunoprecipitation with anti-α7 subunit antibodies (Abs) was used to isolate nAChRs containing α7 subunits from mouse or human brain samples. α7β2-nAChRs were detected in forebrain, but not other tested regions, from both species, based on Western blot analysis of isolates using β2 subunit-specific Abs. Ab specificity was confirmed in control studies using subunit-null mutant mice or cell lines heterologously expressing specific human nAChR subtypes and subunits. Functional expression in Xenopus oocytes of concatenated pentameric (α7)5-, (α7)4(β2)1-, and (α7)3(β2)2-nAChRs was confirmed using two-electrode voltage clamp recording of responses to nicotinic ligands. Importantly, pharmacological profiles were indistinguishable for concatenated (α7)5-nAChRs or for homomeric α7-nAChRs constituted from unlinked α7 subunits. Pharmacological profiles were similar for (α7)5-, (α7)4(β2)1-, and (α7)3(β2)2-nAChRs except for diminished efficacy of nicotine (normalized to acetylcholine efficacy) at α7β2- versus α7-nAChRs. This study represents the first direct confirmation of α7β2-nAChR expression in human and mouse forebrain, supporting previous mouse studies that suggested relevance of α7β2-nAChRs in Alzheimer disease etiopathogenesis. These data also indicate that α7β2-nAChR subunit isoforms with different α7/β2 subunit ratios have similar pharmacological profiles to each other and to α7 homopentameric nAChRs. This supports the hypothesis that α7β2-nAChR agonist activation predominantly or entirely reflects binding to α7/α7 subunit interface sites.
Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


Similar articles
-
α7 and β2 Nicotinic Acetylcholine Receptor Subunits Form Heteromeric Receptor Complexes that Are Expressed in the Human Cortex and Display Distinct Pharmacological Properties.PLoS One. 2015 Jun 18;10(6):e0130572. doi: 10.1371/journal.pone.0130572. eCollection 2015. PLoS One. 2015. PMID: 26086615 Free PMC article.
-
A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides.J Neurosci. 2009 Jan 28;29(4):918-29. doi: 10.1523/JNEUROSCI.3952-08.2009. J Neurosci. 2009. PMID: 19176801 Free PMC article.
-
Unique pharmacology of heteromeric α7β2 nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes.Eur J Pharmacol. 2014 Mar 5;726:77-86. doi: 10.1016/j.ejphar.2014.01.031. Eur J Pharmacol. 2014. PMID: 24485886
-
Heteromeric α7β2 Nicotinic Acetylcholine Receptors in the Brain.Trends Pharmacol Sci. 2016 Jul;37(7):562-574. doi: 10.1016/j.tips.2016.03.005. Epub 2016 May 11. Trends Pharmacol Sci. 2016. PMID: 27179601 Free PMC article. Review.
-
Diverse strategies targeting α7 homomeric and α6β2* heteromeric nicotinic acetylcholine receptors for smoking cessation.Ann N Y Acad Sci. 2014 Oct;1327(1):27-45. doi: 10.1111/nyas.12421. Epub 2014 Apr 14. Ann N Y Acad Sci. 2014. PMID: 24730978 Free PMC article. Review.
Cited by
-
Distinctive single-channel properties of α4β2-nicotinic acetylcholine receptor isoforms.PLoS One. 2019 Mar 7;14(3):e0213143. doi: 10.1371/journal.pone.0213143. eCollection 2019. PLoS One. 2019. PMID: 30845161 Free PMC article.
-
A threshold model for opposing actions of acetylcholine on reward behavior: Molecular mechanisms and implications for treatment of substance abuse disorders.Behav Brain Res. 2016 Oct 1;312:148-62. doi: 10.1016/j.bbr.2016.06.022. Epub 2016 Jun 15. Behav Brain Res. 2016. PMID: 27316344 Free PMC article. Review.
-
In Vitro and In Vivo Characterization of Dibenzothiophene Derivatives [125I]Iodo-ASEM and [18F]ASEM as Radiotracers of Homo- and Heteromeric α7 Nicotinic Acetylcholine Receptors.Molecules. 2020 Mar 20;25(6):1425. doi: 10.3390/molecules25061425. Molecules. 2020. PMID: 32245032 Free PMC article.
-
Molecular function of the novel α7β2 nicotinic receptor.Cell Mol Life Sci. 2018 Jul;75(13):2457-2471. doi: 10.1007/s00018-017-2741-4. Epub 2018 Jan 8. Cell Mol Life Sci. 2018. PMID: 29313059 Free PMC article.
-
Nicotinic ACh receptors as therapeutic targets in CNS disorders.Trends Pharmacol Sci. 2015 Feb;36(2):96-108. doi: 10.1016/j.tips.2014.12.002. Epub 2015 Jan 29. Trends Pharmacol Sci. 2015. PMID: 25639674 Free PMC article. Review.
References
-
- Albuquerque EX, Alkondon M, Pereira EFR, Castro NG, Schrattenholz A, Barbosa CTF, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. (1997) Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther 280:1117–1136 - PubMed
-
- Alkondon M, Pereira EFR, Cortes WS, Maelicke A, Albuquerque EX. (1997) Choline is a selective agonist of α7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 9:2734–2742 - PubMed
-
- Azam L, Winzer-Serhan U, Leslie FM. (2003) Co-expression of α7 and β2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience 119:965–977 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

