Testing for memory-free spectroscopic coordinates by 3D IR exchange spectroscopy
- PMID: 25002483
- PMCID: PMC4115567
- DOI: 10.1073/pnas.1406967111
Testing for memory-free spectroscopic coordinates by 3D IR exchange spectroscopy
Abstract
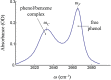
Using 3D infrared (IR) exchange spectroscopy, the ultrafast hydrogen-bond forming and breaking (i.e., complexation) kinetics of phenol to benzene in a benzene/CCl4 mixture is investigated. By introducing a third time point at which the hydrogen-bonding state of phenol is measured (in comparison with 2D IR exchange spectroscopy), the spectroscopic method can serve as a critical test of whether the spectroscopic coordinate used to observe the exchange process is a memory-free, or Markovian, coordinate. For the system under investigation, the 3D IR results suggest that this is not the case. This conclusion is reconfirmed by accompanying molecular dynamics simulations, which furthermore reveal that the non-Markovian kinetics is caused by the heterogeneous structure of the mixed solvent.
Keywords: 3D IR spectroscopy; solvent dynamics; ultrafast dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
What can we learn from three-dimensional infrared spectroscopy?Acc Chem Res. 2009 Sep 15;42(9):1412-22. doi: 10.1021/ar900028k. Acc Chem Res. 2009. PMID: 19449855
-
Hydrogen bond migration between molecular sites observed with ultrafast 2D IR chemical exchange spectroscopy.J Phys Chem B. 2010 Feb 25;114(7):2383-9. doi: 10.1021/jp911452z. J Phys Chem B. 2010. PMID: 20121275 Free PMC article.
-
Phenol-benzene complexation dynamics: quantum chemistry calculation, molecular dynamics simulations, and two dimensional IR spectroscopy.J Chem Phys. 2006 Dec 28;125(24):244508. doi: 10.1063/1.2403132. J Chem Phys. 2006. PMID: 17199356
-
Ultrafast structural molecular dynamics investigated with 2D infrared spectroscopy methods.Top Curr Chem (Cham). 2017 Oct 25;375(6):86. doi: 10.1007/s41061-017-0172-1. Top Curr Chem (Cham). 2017. PMID: 29071445 Review.
-
Two-dimensional infrared spectroscopy of intermolecular hydrogen bonds in the condensed phase.Acc Chem Res. 2009 Sep 15;42(9):1220-8. doi: 10.1021/ar900006u. Acc Chem Res. 2009. PMID: 19425543 Review.
Cited by
-
Migration of small ligands in globins: Xe diffusion in truncated hemoglobin N.PLoS Comput Biol. 2017 Mar 30;13(3):e1005450. doi: 10.1371/journal.pcbi.1005450. eCollection 2017 Mar. PLoS Comput Biol. 2017. PMID: 28358830 Free PMC article.
-
Direct observation of multistep energy transfer in LHCII with fifth-order 3D electronic spectroscopy.Nat Commun. 2015 Jul 31;6:7914. doi: 10.1038/ncomms8914. Nat Commun. 2015. PMID: 26228055 Free PMC article.
References
-
- English BP, et al. Ever-fluctuating single enzyme molecules: Michaelis-Menten equation revisited. Nat Chem Biol. 2006;2(2):87–94. - PubMed
-
- Schmidt-Rohr K, Spiess HW. Nature of nonexponential loss of correlation above the glass transition investigated by multidimensional NMR. Phys Rev Lett. 1991;66(23):3020–3023. - PubMed
-
- Geissler PL, Dellago C, Chandler D. Kinetic pathways of ion pair dissociation in water. J Phys Chem B. 1999;103(18):3706–3710.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

