Folding LacZ in the periplasm of Escherichia coli
- PMID: 25002543
- PMCID: PMC4135689
- DOI: 10.1128/JB.01843-14
Folding LacZ in the periplasm of Escherichia coli
Abstract
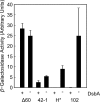
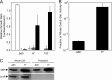
Targeted, translational LacZ fusions provided the initial support for the signal sequence hypothesis in prokaryotes and allowed for selection of the mutations that identified the Sec translocon. Many of these selections relied on the fact that expression of targeted, translational lacZ fusions like malE-lacZ and lamB-lacZ42-1 causes lethal toxicity as folded LacZ jams the translocation pore. However, there is another class of targeted LacZ fusions that do not jam the translocon. These targeted, nonjamming fusions also show toxic phenotypes that may be useful for selecting mutations in genes involved in posttranslocational protein folding and targeting; however, they have not been investigated to the same extent as their jamming counterparts. In fact, it is still unclear whether LacZ can be fully translocated in these fusions. It may be that they simply partition into the inner membrane where they can no longer participate in folding or assembly. In the present study, we systematically characterize the nonjamming fusions and determine their ultimate localization. We report that LacZ can be fully translocated into the periplasm, where it is toxic. We show that this toxicity is likely due to LacZ misfolding and that, in the absence of the periplasmic disulfide bond catalyst DsbA, LacZ folds in the periplasm. Using the novel phenotype of periplasmic β-galactosidase activity, we show that the periplasmic chaperone FkpA contributes to LacZ folding in this nonnative compartment. We propose that targeted, nonjamming LacZ fusions may be used to further study folding and targeting in the periplasm of Escherichia coli.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Activation of colicin M by the FkpA prolyl cis-trans isomerase/chaperone.J Biol Chem. 2011 Feb 25;286(8):6280-90. doi: 10.1074/jbc.M110.165274. Epub 2010 Dec 13. J Biol Chem. 2011. PMID: 21149455 Free PMC article.
-
Effect of folding factors in rescuing unstable heterologous lipase B to enhance its overexpression in the periplasm of Escherichia coli.Appl Microbiol Biotechnol. 2008 Jul;79(6):1035-44. doi: 10.1007/s00253-008-1514-2. Epub 2008 May 22. Appl Microbiol Biotechnol. 2008. PMID: 18496685
-
A system for concomitant overexpression of four periplasmic folding catalysts to improve secretory protein production in Escherichia coli.Protein Eng Des Sel. 2006 Aug;19(8):385-90. doi: 10.1093/protein/gzl018. Epub 2006 May 23. Protein Eng Des Sel. 2006. PMID: 16720693
-
Genetic Analysis of Protein Translocation.Protein J. 2019 Jun;38(3):217-228. doi: 10.1007/s10930-019-09813-y. Protein J. 2019. PMID: 30684070 Free PMC article. Review.
-
Recombinant protein folding and misfolding in Escherichia coli.Nat Biotechnol. 2004 Nov;22(11):1399-408. doi: 10.1038/nbt1029. Nat Biotechnol. 2004. PMID: 15529165 Review.
Cited by
-
FkpA enhances membrane protein folding using an extensive interaction surface.Protein Sci. 2023 Apr;32(4):e4592. doi: 10.1002/pro.4592. Protein Sci. 2023. PMID: 36775935 Free PMC article.
-
The periplasmic chaperone Skp prevents misfolding of the secretory lipase A from Pseudomonas aeruginosa.Front Mol Biosci. 2022 Oct 24;9:1026724. doi: 10.3389/fmolb.2022.1026724. eCollection 2022. Front Mol Biosci. 2022. PMID: 36353734 Free PMC article.
-
Periplasmic β-glucosidase BglX from E. coli demonstrates greater activity towards galactose-containing substrates.Int J Biochem Mol Biol. 2023 Aug 15;14(4):76-86. eCollection 2023. Int J Biochem Mol Biol. 2023. PMID: 37736388 Free PMC article.
-
Development of a sensor for disulfide bond formation in diverse bacteria.J Bacteriol. 2024 Apr 18;206(4):e0043323. doi: 10.1128/jb.00433-23. Epub 2024 Mar 13. J Bacteriol. 2024. PMID: 38493438 Free PMC article.
-
The biogenesis of β-lactamase enzymes.Microbiology (Reading). 2022 Aug;168(8):001217. doi: 10.1099/mic.0.001217. Microbiology (Reading). 2022. PMID: 35943884 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

