Functional antagonism between high temperature requirement protein A (HtrA) family members regulates trophoblast invasion
- PMID: 25002585
- PMCID: PMC4132796
- DOI: 10.1074/jbc.M114.576744
Functional antagonism between high temperature requirement protein A (HtrA) family members regulates trophoblast invasion
Abstract
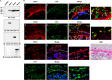
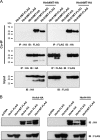
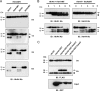
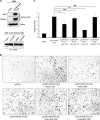
Human trophoblast invasion of decidualized endometrium is essential for placentation and is tightly regulated and involves trophoblast-decidual cell interaction. High temperature requirement A4 (HtrA4) is a secreted serine protease highly expressed in the invasive extravillous trophoblasts that invade decidua. In contrast, both HtrA1 and HtrA3 have been shown to inhibit trophoblast invasion. Here we provide evidence that decidua-secreted HtrA1 and HtrA3 antagonize HtrA4-mediated trophoblast invasion. We demonstrated that HtrA1 and HtrA3 interact with and degrade HtrA4 and thereby inhibit trophoblast-like JAR cell invasion. Specifically, HtrA1 and HtrA3 expression is up-regulated under decidualization conditions in endometrial stromal and epithelial cells, T-HESCs and Ishikawa cells, respectively. Conditioned media from these two cell lines after decidualization treatment suppress HtrA4-expressing JAR cell invasion in an HtrA1- or HtrA3-dependent manner. Co-culture of the HtrA4-expressing JAR cells with decidualization stimuli-treated T-HESC or Ishikawa monolayer also impairs JAR cell invasion, which can be reversed by HtrA1 or HtrA3 knockdown, supporting that HtrA1 and HtrA3 are crucial for trophoblast-decidual cell interaction in the control of trophoblast invasion. Our study reveals a novel regulatory mechanism of trophoblast invasion through physical and functional interaction between HtrA family members.
Keywords: Cell Invasion; Gene Expression; Placenta; Pregnancy; Protease; Trophoblast.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Decidual HtrA3 negatively regulates trophoblast invasion during human placentation.Hum Reprod. 2011 Apr;26(4):748-57. doi: 10.1093/humrep/der019. Epub 2011 Feb 14. Hum Reprod. 2011. PMID: 21321049
-
Extracellular matrix induces trophoblast HtrA4 expression: Implications for the pathogenesis of placenta accreta spectrum.Placenta. 2025 Jun 26;167:71-79. doi: 10.1016/j.placenta.2025.04.028. Epub 2025 May 1. Placenta. 2025. PMID: 40334386
-
Endoplasmic reticulum stress-regulated high temperature requirement A1 (HTRA1) modulates invasion and angiogenesis-related genes in human trophoblasts.J Pharmacol Sci. 2022 Dec;150(4):267-274. doi: 10.1016/j.jphs.2022.10.003. Epub 2022 Oct 7. J Pharmacol Sci. 2022. PMID: 36344049
-
Exploring the Role of HtrA Family Genes in Cancer: A Systematic Review.Mol Diagn Ther. 2024 Jul;28(4):347-377. doi: 10.1007/s40291-024-00712-2. Epub 2024 May 8. Mol Diagn Ther. 2024. PMID: 38717523 Free PMC article.
-
Decidual Control of Trophoblast Invasion.Am J Reprod Immunol. 2016 Mar;75(3):341-50. doi: 10.1111/aji.12466. Epub 2016 Jan 12. Am J Reprod Immunol. 2016. PMID: 26755153 Review.
Cited by
-
Role of microRNAs in trophoblast invasion and spiral artery remodeling: Implications for preeclampsia.Front Cell Dev Biol. 2022 Oct 3;10:995462. doi: 10.3389/fcell.2022.995462. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36263015 Free PMC article. Review.
-
[miR-34a-5p regulates viability, invasion and apoptosis of placental trophoblastic cells via modulating CDK6 and PI3K/AKT pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2020 Jan 30;40(1):79-86. doi: 10.12122/j.issn.1673-4254.2020.01.13. Nan Fang Yi Ke Da Xue Xue Bao. 2020. PMID: 32376568 Free PMC article. Chinese.
-
The Serine Protease HTRA-1 Is a Biomarker for ROP and Mediates Retinal Neovascularization.Front Mol Neurosci. 2020 Nov 17;13:605918. doi: 10.3389/fnmol.2020.605918. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33281553 Free PMC article.
-
Porphyromonas gingivalis-mediated disruption in spiral artery remodeling is associated with altered uterine NK cell populations and dysregulated IL-18 and Htra1.Sci Rep. 2022 Aug 30;12(1):14799. doi: 10.1038/s41598-022-19239-9. Sci Rep. 2022. PMID: 36042379 Free PMC article.
-
HtrA4 Protease Promotes Chemotherapeutic-Dependent Cancer Cell Death.Cells. 2019 Sep 20;8(10):1112. doi: 10.3390/cells8101112. Cells. 2019. PMID: 31546993 Free PMC article.
References
-
- Benirschke K., Kaufmann P. (2001) Nonvillous parts and trophoblast invasion. In Pathology of the Human Placenta, 4th Ed., pp. 171–272, Springer-Verlag, New York
-
- Irwin J. C., Suen L. F., Faessen G. H., Popovici R. M., Giudice L. C. (2001) Insulin-like growth factor (IGF)-II inhibition of endometrial stromal cell tissue inhibitor of metalloproteinase-3 and IGF-binding protein-1 suggests paracrine interactions at the decidua:trophoblast interface during human implantation. J. Clin. Endocrinol. Metab. 86, 2060–2064 - PubMed
-
- Shimonovitz S., Hurwitz A., Dushnik M., Anteby E., Geva-Eldar T., Yagel S. (1994) Developmental regulation of the expression of 72 and 92 kDa type IV collagenases in human trophoblasts: a possible mechanism for control of trophoblast invasion. Am. J. Obstet. Gynecol. 171, 832–838 - PubMed
-
- Damsky C. H., Librach C., Lim K. H., Fitzgerald M. L., McMaster M. T., Janatpour M., Zhou Y., Logan S. K., Fisher S. J. (1994) Integrin switching regulates normal trophoblast invasion. Development 120, 3657–3666 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

