Increasing provision of adolescent vaccines in primary care: a randomized controlled trial
- PMID: 25002671
- PMCID: PMC4531274
- DOI: 10.1542/peds.2013-4257
Increasing provision of adolescent vaccines in primary care: a randomized controlled trial
Abstract
Objectives: To assess the effectiveness of in-person and webinar-delivered AFIX (Assessment, Feedback, Incentives, and eXchange) consultations for increasing adolescent vaccine coverage.
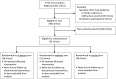
Methods: We randomly assigned 91 primary care clinics in North Carolina, serving 107 443 adolescents, to receive no consultation or an in-person or webinar AFIX consultation. We delivered in-person consultations in April through May 2011 and webinar consultations in May through August 2011. The state's immunization registry provided vaccine coverage data for younger patients (ages 11-12 years) and older patients (ages 13-18 years) for 3 adolescent vaccines: tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap); meningococcal; and human papillomavirus (HPV) vaccines (≥1 dose, females only).
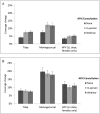
Results: At the 5-month follow-up, AFIX consultations increased vaccine coverage among younger adolescents. Patients in the in-person arm experienced coverage changes that exceeded those in the control arm for Tdap (3.4% [95% confidence interval (CI): 2.2 to 4.6]), meningococcal (4.7% [95% CI: 2.3 to 7.2], and HPV (1.5% [95% CI: 0.3 to 2.7]) vaccines. Patients in the webinar versus control arm also experienced larger changes for these vaccines. AFIX did little to improve coverage among older adolescents. At 1 year, the 3 arms showed similar coverage changes. The effectiveness of in-person and webinar consultations was not statistically different at either time point (all, P >.05).
Conclusions: Webinar AFIX consultations were as effective as in-person consultations in achieving short-term increases in vaccine coverage for younger adolescents. AFIX consultations for adolescents need improvement to have a stronger and more durable impact, especially for HPV vaccine.
Trial registration: ClinicalTrials.gov NCT01544764.
Keywords: North Carolina; adolescent health services; human papillomavirus infections/prevention and control; vaccination/statistics and numerical data.
Copyright © 2014 by the American Academy of Pediatrics.
Figures
References
-
- Centers for Disease Control and Prevention. AFIX (Assessment, Feedback, Incentives, and eXchange). Available at: www.cdc.gov/vaccines/programs/afix/index.html. Accessed October 1, 2013
-
- LeBaron CW, Mercer JT, Massoudi MS, et al. Changes in clinic vaccination coverage after institution of measurement and feedback in 4 states and 2 cities. Arch Pediatr Adolesc Med. 1999;153(8):879–886 - PubMed
-
- Community Preventive Services Task Force. Universally recommended vaccinations: provider assessment and feedback. Available at: www.thecommunityguide.org/vaccines/universally/ providerassessment.html. Accessed March 3, 2014
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous