Progress and hurdles in the development of influenza virus-like particle vaccines for veterinary use
- PMID: 25003086
- PMCID: PMC4083065
- DOI: 10.7774/cevr.2014.3.2.133
Progress and hurdles in the development of influenza virus-like particle vaccines for veterinary use
Abstract
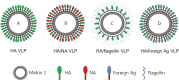
Virus-like particles (VLPs), which resemble infectious virus particles in structure and morphology, have been proposed to provide a new generation of vaccine candidates against various viral infections. As effective immunogens, characterized by high immunogenicity and safety, VLPs have been employed in the development of human influenza vaccines. Recently, several influenza VLP vaccines have been developed for veterinary use and successfully evaluated in swine, canine, duck, and chicken models. These VLP vaccine candidates induced protective immune responses and enabled serological differentiation between vaccinated and infected animals in conjunction with a diagnostic test. Here, we review the current progress of influenza VLP development as a next-generation vaccine technology in the veterinary field and discuss the challenges and future direction of this technology.
Keywords: Influenza; Vaccines; Veterinary; Virus-like particle.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Virus-like particles: potential veterinary vaccine immunogens.Res Vet Sci. 2012 Oct;93(2):553-9. doi: 10.1016/j.rvsc.2011.10.018. Epub 2011 Nov 17. Res Vet Sci. 2012. PMID: 22100244 Review.
-
Development of influenza H7N9 virus like particle (VLP) vaccine: homologous A/Anhui/1/2013 (H7N9) protection and heterologous A/chicken/Jalisco/CPA1/2012 (H7N3) cross-protection in vaccinated mice challenged with H7N9 virus.Vaccine. 2013 Sep 13;31(40):4305-13. doi: 10.1016/j.vaccine.2013.07.043. Epub 2013 Jul 26. Vaccine. 2013. PMID: 23891795
-
Virus-like particle vaccine protects against H3N2 canine influenza virus in dog.Vaccine. 2013 Jul 11;31(32):3268-73. doi: 10.1016/j.vaccine.2013.05.023. Epub 2013 May 22. Vaccine. 2013. PMID: 23707159 Free PMC article.
-
Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge.Viral Immunol. 2005;18(1):244-51. doi: 10.1089/vim.2005.18.244. Viral Immunol. 2005. PMID: 15802970
-
Virus-like particle (VLP)-based vaccines for pandemic influenza: performance of a VLP vaccine during the 2009 influenza pandemic.Hum Vaccin Immunother. 2012 Mar;8(3):411-4. doi: 10.4161/hv.18757. Epub 2012 Feb 14. Hum Vaccin Immunother. 2012. PMID: 22330956 Free PMC article. Review.
Cited by
-
Virus-like particles in poultry disease: an approach to effective and safe vaccination.Front Vet Sci. 2024 Sep 9;11:1405605. doi: 10.3389/fvets.2024.1405605. eCollection 2024. Front Vet Sci. 2024. PMID: 39315089 Free PMC article. Review.
-
Chimeric H5 influenza virus-like particle vaccine elicits broader cross-clade antibody responses in chickens than in ducks.Front Vet Sci. 2023 Jun 15;10:1158233. doi: 10.3389/fvets.2023.1158233. eCollection 2023. Front Vet Sci. 2023. PMID: 37396994 Free PMC article.
-
Influence of temperature on the antigenic changes of virus-like particles.Clin Exp Vaccine Res. 2020 Jul;9(2):126-132. doi: 10.7774/cevr.2020.9.2.126. Epub 2020 Jul 31. Clin Exp Vaccine Res. 2020. PMID: 32864369 Free PMC article.
-
Evaluation of Protective Efficacy of Influenza Virus Like Particles Prepared from H5N1 Virus of Clade 2.2.1.2 in Chickens.Vaccines (Basel). 2021 Jul 1;9(7):715. doi: 10.3390/vaccines9070715. Vaccines (Basel). 2021. PMID: 34358131 Free PMC article.
-
Chimeric Bivalent Virus-Like Particle Vaccine for H5N1 HPAI and ND Confers Protection against a Lethal Challenge in Chickens and Allows a Strategy of Differentiating Infected from Vaccinated Animals (DIVA).PLoS One. 2016 Sep 14;11(9):e0162946. doi: 10.1371/journal.pone.0162946. eCollection 2016. PLoS One. 2016. PMID: 27626934 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

