A single immunization with recombinant rabies virus (ERAG3G) confers complete protection against rabies in mice
- PMID: 25003091
- PMCID: PMC4083070
- DOI: 10.7774/cevr.2014.3.2.176
A single immunization with recombinant rabies virus (ERAG3G) confers complete protection against rabies in mice
Abstract
Purpose: New alternative bait rabies vaccines applicable to pet dogs and wild animals are needed to eradicate rabies in Korea. In this study, recombinant rabies virus, ERAG3G strain was constructed using reverse genetic system and the safety, efficacy and immunogenicity of the ERAG3G strain was evaluated in mice and dogs.
Materials and methods: Using the full-length genome mutated amino acid at position 333 of glycoprotein of rabies virus (RABV) and helper plasmids, the ERAG3G strain was rescued in BHK/T7-9 cells successfully. Mice were inoculated with the ERAG3G strain for safety and efficacy. Safety and immunogenicity of the dog inoculated with the ERAG3G strain (1 mL, 10(8.0) FAID50/mL) via intramuscular route was evaluated for 28 days after inoculation.
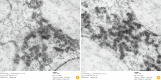
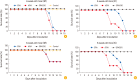
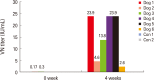
Results: The ERAG3G strain rescued by reverse genetic system was propagated well in the mouse neuroblastoma cells revealing titer of 10(8.5) FAID50/mL and was not pathogenic to 4- or 6-week-old mice that received by intramuscular or intracranical route. Immunization with the ERAG3G strain conferred complete protection from lethal RABV in mice. Dogs inoculated with the vaccine candidate via intramuscular route showed high neutralizing antibody titer ranging from 2.62 to 23.9 IU/mL at 28 days postinoculation.
Conclusion: Our findings suggest that the ERAG3G strain plays an important role in inducing protective efficacy in mice and causes to arise anti-rabies neutralizing antibody in dogs.
Keywords: Animals; Rabies virus; Recombinant rabies virus; Vaccine.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures




Similar articles
-
A recombinant rabies virus (ERAGS) for use in a bait vaccine for swine.Clin Exp Vaccine Res. 2016 Jul;5(2):169-74. doi: 10.7774/cevr.2016.5.2.169. Epub 2016 Jul 29. Clin Exp Vaccine Res. 2016. PMID: 27489807 Free PMC article.
-
Safety and immunogenicity of recombinant rabies virus (ERAGS) in mice and raccoon dogs.Clin Exp Vaccine Res. 2016 Jul;5(2):159-68. doi: 10.7774/cevr.2016.5.2.159. Epub 2016 Jul 29. Clin Exp Vaccine Res. 2016. PMID: 27489806 Free PMC article.
-
Oral immunization of mice with recombinant rabies vaccine strain (ERAG3G) induces complete protection.Clin Exp Vaccine Res. 2015 Jan;4(1):107-13. doi: 10.7774/cevr.2015.4.1.107. Epub 2015 Jan 30. Clin Exp Vaccine Res. 2015. PMID: 25648184 Free PMC article.
-
Application of recombinant adenoviruses expressing glycoprotein or nucleoprotein of rabies virus to Korean raccoon dogs.Clin Exp Vaccine Res. 2015 Jul;4(2):189-94. doi: 10.7774/cevr.2015.4.2.189. Epub 2015 Jul 29. Clin Exp Vaccine Res. 2015. PMID: 26273578 Free PMC article.
-
Genetically modified rabies virus ERA strain is safe and induces long-lasting protective immune response in dogs after oral vaccination.Antiviral Res. 2015 Sep;121:9-15. doi: 10.1016/j.antiviral.2015.06.011. Epub 2015 Jun 18. Antiviral Res. 2015. PMID: 26093157
Cited by
-
A recombinant rabies virus (ERAGS) for use in a bait vaccine for swine.Clin Exp Vaccine Res. 2016 Jul;5(2):169-74. doi: 10.7774/cevr.2016.5.2.169. Epub 2016 Jul 29. Clin Exp Vaccine Res. 2016. PMID: 27489807 Free PMC article.
-
Safety and immunogenicity of recombinant rabies virus (ERAGS) in mice and raccoon dogs.Clin Exp Vaccine Res. 2016 Jul;5(2):159-68. doi: 10.7774/cevr.2016.5.2.159. Epub 2016 Jul 29. Clin Exp Vaccine Res. 2016. PMID: 27489806 Free PMC article.
-
Establishment of a reverse genetics system for rabies virus strain Komatsugawa.J Vet Med Sci. 2022 Nov 7;84(11):1508-1513. doi: 10.1292/jvms.22-0254. Epub 2022 Sep 29. J Vet Med Sci. 2022. PMID: 36171109 Free PMC article.
-
Oral immunization of mice with recombinant rabies vaccine strain (ERAG3G) induces complete protection.Clin Exp Vaccine Res. 2015 Jan;4(1):107-13. doi: 10.7774/cevr.2015.4.1.107. Epub 2015 Jan 30. Clin Exp Vaccine Res. 2015. PMID: 25648184 Free PMC article.
References
-
- Johnson N, Black C, Smith J, et al. Rabies emergence among foxes in Turkey. J Wildl Dis. 2003;39:262–270. - PubMed
-
- Yang DK, Kim SY, Oh YI, et al. Epidemiological characteristics of rabies in South Korea from January 2004 to March 2011. J Bacteriol Virol. 2011;41:165–171.
-
- Orciari LA, Niezgoda M, Hanlon CA, et al. Rapid clearance of SAG-2 rabies virus from dogs after oral vaccination. Vaccine. 2001;19:4511–4518. - PubMed
-
- Faber M, Dietzschold B, Li J. Immunogenicity and safety of recombinant rabies viruses used for oral vaccination of stray dogs and wildlife. Zoonoses Public Health. 2009;56:262–269. - PubMed
-
- Baer GM, Abelseth MK, Debbie JG. Oral vaccination of foxes against rabies. Am J Epidemiol. 1971;93:487–490. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

