Erythrophagocytosis in Entamoeba histolytica and Entamoeba dispar: a comparative study
- PMID: 25003123
- PMCID: PMC4066688
- DOI: 10.1155/2014/626259
Erythrophagocytosis in Entamoeba histolytica and Entamoeba dispar: a comparative study
Abstract
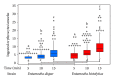
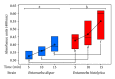
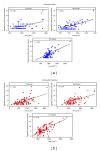
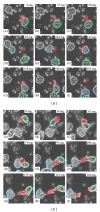
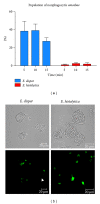
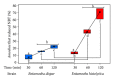
Entamoeba histolytica is the causative agent of human intestinal and liver amebiasis. The extraordinary phagocytic activity of E. histolytica trophozoites has been accepted as one of the virulence mechanisms responsible for their invasive capacity. The recognition of the noninvasive Entamoeba dispar as a different species has raised the question as to whether the lack of pathogenic potential of this ameba correlates with a limited phagocytic capacity. We have therefore compared the process of erythrophagocytosis in both species by means of light and video microscopy, hemoglobin measurement, and the estimation of reactive oxygen species (ROS). In the present study, we confirmed that E. dispar has lower erythrophagocytic capacity. We also observed by video microscopy a new event of erythrocyte opsonization-like in both species, being more characteristic in E. histolytica. Moreover, E. dispar showed a lower capacity to produce ROS compared with the invasive species and also showed a large population of amoebae that did not engulf any erythrocyte over time. Our results demonstrate that E. histolytica has a higher phagocytic capacity than E. dispar, including a higher rate of production of ROS in the course of ingesting red blood cells.
Figures

References
-
- World Health Organization. Amoebiasis. Weekly Epidemiological Record. 1997;72:97–98. - PubMed
-
- Haque R, Huston CD, Hughes M, Houpt E, Petri WA., Jr. Amebiasis. The New England Journal of Medicine. 2003;348(16):1565–1573. - PubMed
-
- Martínez-Palomo A. Parasitic amebas of the intestinal tract. In: Kreier JP, Barker JR, editors. Parasitic Protozoa. 2nd edition. New York, NY, USA: Academic Press; 1993. pp. 65–141.
-
- WHO/PAHO/UNESCO report. A consultation with experts on amoebiasis. Mexico City, Mexico 28-29 January, 1997. Epidemiological Bulletin. 1997;18(1):13–14. - PubMed
-
- Diamond LS, Clark CG. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. The Journal of Eukaryotic Microbiology. 1993;40(3):340–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

