Multicenter closed-loop insulin delivery study points to challenges for keeping blood glucose in a safe range by a control algorithm in adults and adolescents with type 1 diabetes from various sites
- PMID: 25003311
- PMCID: PMC4183913
- DOI: 10.1089/dia.2014.0066
Multicenter closed-loop insulin delivery study points to challenges for keeping blood glucose in a safe range by a control algorithm in adults and adolescents with type 1 diabetes from various sites
Erratum in
-
Correction.Diabetes Technol Ther. 2015 Jan;17(1):68. doi: 10.1089/dia.2014.1610. Diabetes Technol Ther. 2015. PMID: 25574594 Free PMC article. No abstract available.
Abstract
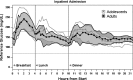
Background: The Control to Range Study was a multinational artificial pancreas study designed to assess the time spent in the hypo- and hyperglycemic ranges in adults and adolescents with type 1 diabetes while under closed-loop control. The controller attempted to keep the glucose ranges between 70 and 180 mg/dL. A set of prespecified metrics was used to measure safety.
Research design and methods: We studied 53 individuals for approximately 22 h each during clinical research center admissions. Plasma glucose level was measured every 15-30 min (YSI clinical laboratory analyzer instrument [YSI, Inc., Yellow Springs, OH]). During the admission, subjects received three mixed meals (1 g of carbohydrate/kg of body weight; 100 g maximum) with meal announcement and automated insulin dosing by the controller.
Results: For adults, the mean of subjects' mean glucose levels was 159 mg/dL, and mean percentage of values 71-180 mg/dL was 66% overall (59% daytime and 82% overnight). For adolescents, the mean of subjects' mean glucose levels was 166 mg/dL, and mean percentage of values in range was 62% overall (53% daytime and 82% overnight). Whereas prespecified criteria for safety were satisfied by both groups, they were met at the individual level in adults only for combined daytime/nighttime and for isolated nighttime. Two adults and six adolescents failed to meet the daytime criterion, largely because of postmeal hyperglycemia, and another adolescent failed to meet the nighttime criterion.
Conclusions: The control-to-range system performed as expected: faring better overnight than during the day and performing with variability between patients even after individualization based on patients' prior settings. The system had difficulty preventing postmeal excursions above target range.
Trial registration: ClinicalTrials.gov NCT01271023.
Figures
References
-
- Juvenile Diabetes Research Foundation: Emerging technologies in diabetes research. The JDRF e-Newsletter 2006; (Sept):10
-
- O'Grady MJ, Retterath AJ, Keenan DB, Kurtz N, Cantwell M, Spital G, Kremliovsky MN, Roy A, Davis EA, Jones TW, Ly TT: The use of an automated, portable glucose control system for overnight glucose control in adolescents and young adults with type 1 diabetes. Diabetes Care 2012;35:2182–2187 - PMC - PubMed
-
- Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF: Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes 2006;55:3344–3350 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous