Immunomodulatory effects of bone marrow-derived mesenchymal stem cells on pro-inflammatory cytokine-stimulated human corneal epithelial cells
- PMID: 25003339
- PMCID: PMC4086952
- DOI: 10.1371/journal.pone.0101841
Immunomodulatory effects of bone marrow-derived mesenchymal stem cells on pro-inflammatory cytokine-stimulated human corneal epithelial cells
Abstract
Purpose: To investigate the modulatory effect of rat bone marrow mesenchymal stem cells (MSC) on human corneal epithelial cells (HCE-T) stimulated with pro-inflammatory cytokines interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) in an in vitro co-cultured model.
Methods: HCE-T alone and co-cultured with MSC were stimulated with IFN-γ/TNF for 24 and 48 hours or left untreated. The expression of intracellular adhesion molecule (ICAM)-1, human leukocyte antigen ABC, DR and G (HLA-ABC, HLA-DR, HLA-G) were investigated by flow cytometry. Subcellular localization of nuclear factor-kappa B (NF-κB) and expression of indoleamine 2,3-dioxygenase (IDO) were assessed by immunofluorescence staining and western blot. The concentration of transforming growth factor beta 1 (TGF-β1) in the conditioned media from different cultures was evaluated by enzyme-linked immunosorbent assay. NF-κB and TGF-β1 signaling pathway blocking experiments were performed to analyze associations between the expression of cell surface molecules and the NF-κB transcription pathway, and the expression of IDO and TGF-β1 signaling pathway.
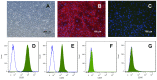
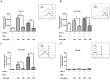
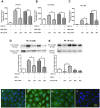
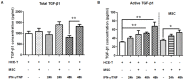
Results: IFN-γ/TNF treatment significantly up-regulated expression of ICAM-1, HLA-ABC, and induced de novo expression of HLA-DR and IDO on HCE-T cultured alone, while HLA-G expression remained unaffected. Up-regulation was significantly inhibited by co-culture with MSC. Increased TGF-β1 secretion was detected in 48 h IFN-γ/TNF-stimulated MSC monocultures and HCE-T/MSC co-cultures. MSC attenuated the activation of cytokine-induced NF-κB and IDO induction. Blockade of NF-κB transcription pathway by BMS-345541 significantly reduced the up-regulation of ICAM-1, HLA-ABC, HLA-DR and IDO expression, while blockade of TGF-β1 signaling pathways reversed the modulatory effect of MSC on IDO expression.
Conclusions: MSC reduced the expression of adhesion and immunoregulatory molecules on pro-inflammatory cytokine-stimulated HCE-T via the NF-κB transcription pathway. MSC attenuated expression of IDO through both NF-κB transcription and TGF-β1 signaling pathways. Co-culture of HCEC with MSC therefore provides a useful in vitro model to study the anti-inflammatory properties of MSC on corneal epithelium.
Conflict of interest statement
Figures


Similar articles
-
IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells.Immunol Lett. 2007 Jun 15;110(2):91-100. doi: 10.1016/j.imlet.2007.04.001. Epub 2007 Apr 26. Immunol Lett. 2007. PMID: 17507101
-
Effect of TGF-β on ocular surface epithelial cells.Exp Eye Res. 2013 Feb;107:88-100. doi: 10.1016/j.exer.2012.11.017. Epub 2012 Dec 7. Exp Eye Res. 2013. PMID: 23220729
-
An immunoprotective privilege of corneal epithelial stem cells against Th17 inflammatory stress by producing glial cell-derived neurotrophic factor.Stem Cells. 2010 Dec;28(12):2172-81. doi: 10.1002/stem.539. Stem Cells. 2010. PMID: 20936708 Free PMC article.
-
TNF-α and IFN-γ Participate in Improving the Immunoregulatory Capacity of Mesenchymal Stem/Stromal Cells: Importance of Cell-Cell Contact and Extracellular Vesicles.Int J Mol Sci. 2021 Sep 2;22(17):9531. doi: 10.3390/ijms22179531. Int J Mol Sci. 2021. PMID: 34502453 Free PMC article. Review.
-
Redressing the interactions between stem cells and immune system in tissue regeneration.Biol Direct. 2021 Oct 20;16(1):18. doi: 10.1186/s13062-021-00306-6. Biol Direct. 2021. PMID: 34670590 Free PMC article. Review.
Cited by
-
Concise Review: Bioengineering of Limbal Stem Cell Niche.Bioengineering (Basel). 2023 Jan 12;10(1):111. doi: 10.3390/bioengineering10010111. Bioengineering (Basel). 2023. PMID: 36671683 Free PMC article. Review.
-
Ruxolitinib inhibits IFNγ-stimulated Sjögren's salivary gland MSC HLA-DR expression and chemokine-dependent T cell migration.Rheumatology (Oxford). 2022 Oct 6;61(10):4207-4218. doi: 10.1093/rheumatology/keac111. Rheumatology (Oxford). 2022. PMID: 35218354 Free PMC article.
-
Dectin-1 agonist curdlan modulates innate immunity to Aspergillus fumigatus in human corneal epithelial cells.Int J Ophthalmol. 2015 Aug 18;8(4):690-6. doi: 10.3980/j.issn.2222-3959.2015.04.09. eCollection 2015. Int J Ophthalmol. 2015. PMID: 26309863 Free PMC article.
-
Development of In Vitro Dry Eye Models to Study Proliferative and Anti-Inflammatory Effects of Allogeneic Serum Eye Drops.Int J Mol Sci. 2023 Jan 13;24(2):1567. doi: 10.3390/ijms24021567. Int J Mol Sci. 2023. PMID: 36675083 Free PMC article.
-
Immunological priming of mesenchymal stromal/stem cells and their extracellular vesicles augments their therapeutic benefits in experimental graft-versus-host disease via engagement of PD-1 ligands.Front Immunol. 2023 Feb 16;14:1078551. doi: 10.3389/fimmu.2023.1078551. eCollection 2023. Front Immunol. 2023. PMID: 36875112 Free PMC article.
References
-
- Oh JY, Kim MK, Shin MS, Lee HJ, Lee JH, et al. (2009) The anti-inflammatory effect of subconjunctival bevacizumab on chemically burned rat corneas. Curr Eye Res 34: 85–91. - PubMed
-
- Uwaydat S, Jha P, Tytarenko R, Brown H, Wiggins M, et al. (2011) The use of topical honey in the treatment of corneal abrasions and endotoxin-induced keratitis in an animal model. Curr Eye Res 36: 787–796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

