β-Catenin inactivation is a pre-requisite for chick retina regeneration
- PMID: 25003522
- PMCID: PMC4086939
- DOI: 10.1371/journal.pone.0101748
β-Catenin inactivation is a pre-requisite for chick retina regeneration
Abstract
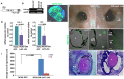
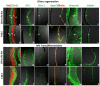
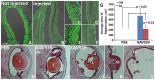
In the present study we explored the role of β-catenin in mediating chick retina regeneration. The chick can regenerate its retina by activating stem/progenitor cells present in the ciliary margin (CM) of the eye or via transdifferentiation of the retinal pigmented epithelium (RPE). Both modes require fibroblast growth factor 2 (FGF2). We observed, by immunohistochemistry, dynamic changes of nuclear β-catenin in the CM and RPE after injury (retinectomy). β-Catenin nuclear accumulation was transiently lost in cells of the CM in response to injury alone, while the loss of nuclear β-catenin was maintained as long as FGF2 was present. However, nuclear β-catenin positive cells remained in the RPE in response to injury and were BrdU-/p27+, suggesting that nuclear β-catenin prevents those cells from entering the cell cycle. If FGF2 is present, the RPE undergoes dedifferentiation and proliferation concomitant with loss of nuclear β-catenin. Moreover, retinectomy followed by disruption of active β-catenin by using a signaling inhibitor (XAV939) or over-expressing a dominant negative form of Lef-1 induces regeneration from both the CM and RPE in the absence of FGF2. Our results imply that β-catenin protects cells of the CM and RPE from entering the cell cycle in the developing eye, and specifically for the RPE during injury. Thus inactivation of β-catenin is a pre-requisite for chick retina regeneration.
Conflict of interest statement
Figures





Similar articles
-
Reprogramming of the chick retinal pigmented epithelium after retinal injury.BMC Biol. 2014 Apr 17;12:28. doi: 10.1186/1741-7007-12-28. BMC Biol. 2014. PMID: 24742279 Free PMC article.
-
Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression.Development. 2009 Aug;136(15):2505-10. doi: 10.1242/dev.032136. Epub 2009 Jun 24. Development. 2009. PMID: 19553286 Free PMC article.
-
β-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina.Neural Dev. 2012 Aug 24;7:30. doi: 10.1186/1749-8104-7-30. Neural Dev. 2012. PMID: 22920725 Free PMC article.
-
Growth factor-induced retinal regeneration in vivo.Int Rev Cytol. 1993;146:49-74. doi: 10.1016/s0074-7696(08)60379-4. Int Rev Cytol. 1993. PMID: 8360013 Review.
-
Wnt/β-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson's Disease.Int J Mol Sci. 2018 Nov 24;19(12):3743. doi: 10.3390/ijms19123743. Int J Mol Sci. 2018. PMID: 30477246 Free PMC article. Review.
Cited by
-
Transcriptome Profiling of Embryonic Retinal Pigment Epithelium Reprogramming.Genes (Basel). 2021 May 29;12(6):840. doi: 10.3390/genes12060840. Genes (Basel). 2021. PMID: 34072522 Free PMC article.
-
Wnt/β-catenin signaling pathway regulates cell proliferation but not muscle dedifferentiation nor apoptosis during sea cucumber intestinal regeneration.Dev Biol. 2021 Dec;480:105-113. doi: 10.1016/j.ydbio.2021.08.011. Epub 2021 Sep 3. Dev Biol. 2021. PMID: 34481794 Free PMC article.
-
The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems.Prog Retin Eye Res. 2021 Nov;85:100969. doi: 10.1016/j.preteyeres.2021.100969. Epub 2021 Apr 23. Prog Retin Eye Res. 2021. PMID: 33901682 Free PMC article. Review.
-
Electroporation of Embryonic Chick Eyes.Bio Protoc. 2015 Jun 20;5(12):e1498. doi: 10.21769/bioprotoc.1498. Bio Protoc. 2015. PMID: 27054146 Free PMC article.
-
Cell Sources for Retinal Regeneration: Implication for Data Translation in Biomedicine of the Eye.Cells. 2022 Nov 24;11(23):3755. doi: 10.3390/cells11233755. Cells. 2022. PMID: 36497013 Free PMC article. Review.
References
-
- Haynes T, Del Rio-Tsonis K (2004) Retina repair, stem cells and beyond. Curr Neurovasc Res 1: 231–239. - PubMed
-
- Barbosa-Sabanero K, Hoffmann A, Judge C, Lightcap N, Tsonis PA, et al. (2012) Lens and retina regeneration: new perspectives from model organisms. Biochem J 447: 321–334. - PubMed
-
- Chiba C (2014) The retinal pigment epithelium: An important player of retinal disorders and regeneration. Exp Eye Res 123: 107–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

