Uremic conditions drive human monocytes to pro-atherogenic differentiation via an angiotensin-dependent mechanism
- PMID: 25003524
- PMCID: PMC4087008
- DOI: 10.1371/journal.pone.0102137
Uremic conditions drive human monocytes to pro-atherogenic differentiation via an angiotensin-dependent mechanism
Abstract
Aims: Elevated expression levels of monocytic-ACE have been found in haemodialysis patients. They are not only epidemiologically linked with increased mortality and cardiovascular disease, but may also directly participate in the initial steps of atherosclerosis. To further address this question we tested the role of monocytic-ACE in promotion of atherosclerotic events in vitro under conditions mimicking those of chronic renal failure.
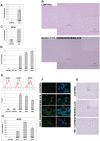
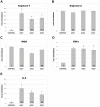
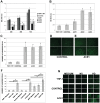
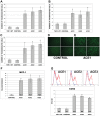
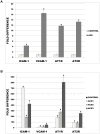
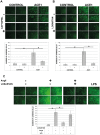
Methods and results: Treatment of human primary monocytes or THP-1 cells with uremic serum as well as PMA-induced differentiation led to significantly up-regulated expression of ACE, further increased by additional treatment with LPS. Functionally, these monocytes revealed significantly increased adhesion and transmigration through endothelial monolayers. Overexpression of ACE in transfected monocytes or THP-1 cells led to development of more differentiated, macrophage-like phenotype with up-regulated expression of Arg1, MCSF, MCP-1 and CCR2. Expression of pro-inflammatory cytokines TNFa and IL-6 were also noticeably up-regulated. ACE overexpression resulted in significantly increased adhesion and transmigration properties. Transcriptional screening of ACE-overexpressing monocytes revealed noticeably increased expression of Angiotensin II receptors and adhesion- as well as atherosclerosis-related ICAM-1 and VCAM1. Inhibition of monocyte ACE or AngII-receptor signalling led to decreased adhesion potential of ACE-overexpressing cells.
Conclusions: Taken together, these data demonstrate that uremia induced expression of monocytic-ACE mediates the development of highly pro-atherogenic cells via an AngII-dependent mechanism.
Conflict of interest statement
Figures


References
-
- Foley RN, Parfrey PS, Sarnak MJ (1998) Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney 32: 112–119. - PubMed
-
- Cheung AK, Sarnak MJ, Yan G, Dwyer JT, Heyka RJ, et al. (2000) Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int 58: 353–362. - PubMed
-
- Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, et al. (2005) Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 20: 1048–1056. - PubMed
-
- Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, Woodward M, et al. (2010) Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 12: 2073–2081. - PMC - PubMed
-
- Tyralla K, Amann K (2003) Morphology of the heart and arteries in renal failure. Kidney Int Suppl 84: 80–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

