A virtual screen identified C96 as a novel inhibitor of phosphatidylinositol 3-kinase that displays potent preclinical activity against multiple myeloma in vitro and in vivo
- PMID: 25003534
- PMCID: PMC4116524
- DOI: 10.18632/oncotarget.1657
A virtual screen identified C96 as a novel inhibitor of phosphatidylinositol 3-kinase that displays potent preclinical activity against multiple myeloma in vitro and in vivo
Abstract
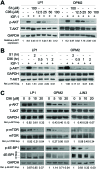
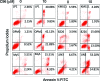
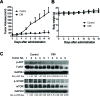
The phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway is emerging as a promising therapeutic target for multiple myeloma (MM). In the present study, we performed a virtual screen against 800,000 of small molecule compounds by targeting PI3Kγ. C96, one of such compounds, inhibited PI3K activated by insulin-like growth factor-1 (IGF-1), but did not suppress IGF-1R activation. The cell-free assay revealed that C96 preferred to inhibit PI3Kα and δ, but was not active against AKT1, 2, 3 or mTOR. C96 inhibited PI3K activation in a time- and concentration-dependent manner. Consistent with its inhibition on PI3K/AKT, C96 downregulated the activation of mTOR, p70S6K, 4E-BP1, but did not suppress other kinases such as ERK and c-Src. Inhibition of the PI3K/AKT signaling pathway by C96 led to MM cell apoptosis which was demonstrated by Annexin V staining and activation of the pro-apoptotic signals. Furthermore, C96 displayed potent anti-myeloma activity in a MM xenograft model in nude mice. Oral administration of 100 mg/kg bodyweight almost fully suppressed tumor growth within 16 days, but without gross toxicity. Importantly, AKT activation was suppressed in tumor tissues from C96-treated mice, which was consistent with delayed tumor growth. Thus, we identified a novel PI3K inhibitor with a great potential for MM therapy.
Conflict of interest statement
There is no conflict of interest to declare for this study.
Figures




Similar articles
-
A novel PI3K inhibitor PIK-C98 displays potent preclinical activity against multiple myeloma.Oncotarget. 2015 Jan 1;6(1):185-95. doi: 10.18632/oncotarget.2688. Oncotarget. 2015. PMID: 25474140 Free PMC article.
-
Identification of a promising PI3K inhibitor for the treatment of multiple myeloma through the structural optimization.J Hematol Oncol. 2014 Jan 15;7:9. doi: 10.1186/1756-8722-7-9. J Hematol Oncol. 2014. PMID: 24428908 Free PMC article.
-
Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade.Mol Cancer Ther. 2005 Oct;4(10):1533-40. doi: 10.1158/1535-7163.MCT-05-0068. Mol Cancer Ther. 2005. PMID: 16227402
-
Antimyeloma activity of the orally bioavailable dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235.Cancer Res. 2009 Jul 15;69(14):5835-42. doi: 10.1158/0008-5472.CAN-08-4285. Epub 2009 Jul 7. Cancer Res. 2009. PMID: 19584292
-
Targeting the phosphatidylinositol 3-kinase/AKT pathway for the treatment of multiple myeloma.Curr Med Chem. 2014;21(27):3173-87. doi: 10.2174/0929867321666140601204513. Curr Med Chem. 2014. PMID: 24934342 Review.
Cited by
-
Tanshinone IIA Attenuates Insulin Like Growth Factor 1 -Induced Cell Proliferation in PC12 Cells through the PI3K/Akt and MEK/ERK Pathways.Int J Mol Sci. 2018 Sep 12;19(9):2719. doi: 10.3390/ijms19092719. Int J Mol Sci. 2018. PMID: 30213025 Free PMC article.
-
Future of Personalized Therapy Targeting Aberrant Signaling Pathways in Multiple Myeloma.Clin Lymphoma Myeloma Leuk. 2019 Jul;19(7):397-405. doi: 10.1016/j.clml.2019.03.017. Epub 2019 Mar 25. Clin Lymphoma Myeloma Leuk. 2019. PMID: 31036508 Free PMC article. Review.
-
Synergistic cytotoxic effects of bortezomib and CK2 inhibitor CX-4945 in acute lymphoblastic leukemia: turning off the prosurvival ER chaperone BIP/Grp78 and turning on the pro-apoptotic NF-κB.Oncotarget. 2016 Jan 12;7(2):1323-40. doi: 10.18632/oncotarget.6361. Oncotarget. 2016. PMID: 26593250 Free PMC article.
-
A novel PI3K inhibitor PIK-C98 displays potent preclinical activity against multiple myeloma.Oncotarget. 2015 Jan 1;6(1):185-95. doi: 10.18632/oncotarget.2688. Oncotarget. 2015. PMID: 25474140 Free PMC article.
-
Liposomes have a direct effect on multiple myeloma: a Mendelian randomization study.Front Oncol. 2024 Jun 11;14:1404744. doi: 10.3389/fonc.2024.1404744. eCollection 2024. Front Oncol. 2024. PMID: 38933448 Free PMC article.
References
-
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. - PubMed
-
- Cao B, Li J, Mao X. Dissecting bortezomib: development, application, adverse effects and future direction. Curr Pharm Des. 2013;19(18):3190–3200. - PubMed
-
- Herndon TM, Deisseroth A, Kaminskas E, Kane RC, Koti KM, Rothmann MD, Habtemariam B, Bullock J, Bray JD, Hawes J, et al. U.s. Food and drug administration approval: carfilzomib for the treatment of multiple myeloma. Clin Cancer Res. 2013;19(17):4559–4563. - PubMed
-
- Li J, Cao B, Zhou S, Zhu J, Zhang Z, Hou T, Mao X. Cyproheptadine-induced myeloma cell apoptosis is associated with inhibition of the PI3K/AKT signaling. Eur J Haematol. 2013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

