Inhibition mechanism of the intracellular transporter Ca2+-pump from sarco-endoplasmic reticulum by the antitumor agent dimethyl-celecoxib
- PMID: 25003576
- PMCID: PMC4086972
- DOI: 10.1371/journal.pone.0102083
Inhibition mechanism of the intracellular transporter Ca2+-pump from sarco-endoplasmic reticulum by the antitumor agent dimethyl-celecoxib
Abstract
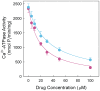
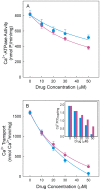
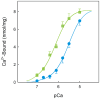
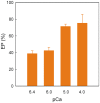
Dimethyl-celecoxib is a celecoxib analog that lacks the capacity as cyclo-oxygenase-2 inhibitor and therefore the life-threatening effects but retains the antineoplastic properties. The action mechanism at the molecular level is unclear. Our in vitro assays using a sarcoplasmic reticulum preparation from rabbit skeletal muscle demonstrate that dimethyl-celecoxib inhibits Ca2+-ATPase activity and ATP-dependent Ca2+ transport in a concentration-dependent manner. Celecoxib was a more potent inhibitor of Ca2+-ATPase activity than dimethyl-celecoxib, as deduced from the half-maximum effect but dimethyl-celecoxib exhibited higher inhibition potency when Ca2+ transport was evaluated. Since Ca2+ transport was more sensitive to inhibition than Ca2+-ATPase activity the drugs under study caused Ca2+/Pi uncoupling. Dimethyl-celecoxib provoked greater uncoupling and the effect was dependent on drug concentration but independent of Ca2+-pump functioning. Dimethyl-celecoxib prevented Ca2+ binding by stabilizing the inactive Ca2+-free conformation of the pump. The effect on the kinetics of phosphoenzyme accumulation and the dependence of the phosphoenzyme level on dimethyl-celecoxib concentration were independent of whether or not the Ca2+-pump was exposed to the drug in the presence of Ca2+ before phosphorylation. This provided evidence of non-preferential interaction with the Ca2+-free conformation. Likewise, the decreased phosphoenzyme level in the presence of dimethyl-celecoxib that was partially relieved by increasing Ca2+ was consistent with the mentioned effect on Ca2+ binding. The kinetics of phosphoenzyme decomposition under turnover conditions was not altered by dimethyl-celecoxib. The dual effect of the drug involves Ca2+-pump inhibition and membrane permeabilization activity. The reported data can explain the cytotoxic and anti-proliferative effects that have been attributed to the celecoxib analog. Ligand docking simulation predicts interaction of celecoxib and dimethyl-celecoxib with the intracellular Ca2+ transporter at the inhibition site of hydroquinones.
Conflict of interest statement
Figures




References
-
- Smith WL, Garavito RM, DeWitt DL (1996) Prostaglandin endoperoxide H syntases (cyclooxygenases)-1 and -2. J Biol Chem 271: 33157–33160. - PubMed
-
- Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, et al. (1997) Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, celecoxib). J Med Chem 40: 1347–1365. - PubMed
-
- Reddy BS, Rao CV, Seibert K (1996) Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventive properties in colon carcinogenesis. Cancer Res 56: 4566–45669. - PubMed
-
- Harris RE, Alshafie GA, Abou-Issa H, Seibert K (2000) Chemoprevention of breast cancer in rats with celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res 60: 2101–2103. - PubMed
-
- Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, et al. (1994) Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 107: 1183–1188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

