Melanoma cell therapy: Endothelial progenitor cells as shuttle of the MMP12 uPAR-degrading enzyme
- PMID: 25003596
- PMCID: PMC4116515
- DOI: 10.18632/oncotarget.1987
Melanoma cell therapy: Endothelial progenitor cells as shuttle of the MMP12 uPAR-degrading enzyme
Abstract
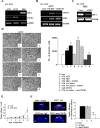
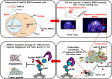
The receptor for the urokinase-type plasminogen activator (uPAR) accounts for many features of cancer progression, and is therefore considered a target for anti-tumoral therapy. Only full length uPAR mediates tumor progression. Matrix-metallo-proteinase-12 (MMP12)-dependent uPAR cleavage results into the loss of invasion properties and angiogenesis. MMP12 can be employed in the field of "targeted therapies" as a biological drug to be delivered directly in patient's tumor mass. Endothelial Progenitor Cells (EPCs) are selectively recruited within the tumor and could be used as cellular vehicles for delivering anti-cancer molecules. The aim of our study is to inhibit cancer progression by engeneering ECFCs, a subset of EPC, with a lentivirus encoding the anti-tumor uPAR-degrading enzyme MMP12. Ex vivo manipulated ECFCs lost the capacity to perform capillary morphogenesis and acquired the anti-tumor and anti-angiogenetic activity. In vivo MMP12-engineered ECFCs cleaved uPAR within the tumor mass and strongly inhibited tumor growth, tumor angiogenesis and development of lung metastasis. The possibility to exploit tumor homing and activity of autologous MMP12-engineered ECFCs represents a novel way to combat melanoma by a "personalized therapy", without rejection risk. The i.v. injection of radiolabelled MMP12-ECFCs can thus provide a new theranostic approach to control melanoma progression and metastasis.
Figures





Similar articles
-
Endothelial progenitor cell-dependent angiogenesis requires localization of the full-length form of uPAR in caveolae.Blood. 2011 Sep 29;118(13):3743-55. doi: 10.1182/blood-2011-02-338681. Epub 2011 Jul 29. Blood. 2011. PMID: 21803847
-
Modulation of the angiogenic phenotype of normal and systemic sclerosis endothelial cells by gain-loss of function of pentraxin 3 and matrix metalloproteinase 12.Arthritis Rheum. 2010 Aug;62(8):2488-98. doi: 10.1002/art.27522. Arthritis Rheum. 2010. PMID: 20506099
-
Cleavage of the urokinase receptor (uPAR) on oral cancer cells: regulation by transforming growth factor - β1 (TGF-β1) and potential effects on migration and invasion.BMC Cancer. 2017 May 19;17(1):350. doi: 10.1186/s12885-017-3349-7. BMC Cancer. 2017. PMID: 28526008 Free PMC article.
-
The many spaces of uPAR: delivery of theranostic agents and nanobins to multiple tumor compartments through a single target.Theranostics. 2013 Jun 25;3(7):496-506. doi: 10.7150/thno.4953. Print 2013. Theranostics. 2013. PMID: 23843897 Free PMC article. Review.
-
Urokinase-type plasminogen activator (uPA) and its receptor (uPAR): development of antagonists of uPA/uPAR interaction and their effects in vitro and in vivo.Curr Pharm Des. 2003;9(19):1529-43. doi: 10.2174/1381612033454612. Curr Pharm Des. 2003. PMID: 12871066 Review.
Cited by
-
uPAR: An Essential Factor for Tumor Development.J Cancer. 2021 Oct 17;12(23):7026-7040. doi: 10.7150/jca.62281. eCollection 2021. J Cancer. 2021. PMID: 34729105 Free PMC article. Review.
-
CRISPR/Cas9 uPAR Gene Knockout Results in Tumor Growth Inhibition, EGFR Downregulation and Induction of Stemness Markers in Melanoma and Colon Carcinoma Cell Lines.Front Oncol. 2021 May 14;11:663225. doi: 10.3389/fonc.2021.663225. eCollection 2021. Front Oncol. 2021. PMID: 34055629 Free PMC article.
-
Endothelial progenitor cells in tumor angiogenesis: another brick in the wall.Stem Cells Int. 2015;2015:832649. doi: 10.1155/2015/832649. Epub 2015 Apr 27. Stem Cells Int. 2015. PMID: 26000021 Free PMC article. Review.
-
Enhanced Vasculogenic Capacity Induced by 5-Fluorouracil Chemoresistance in a Gastric Cancer Cell Line.Int J Mol Sci. 2021 Jul 19;22(14):7698. doi: 10.3390/ijms22147698. Int J Mol Sci. 2021. PMID: 34299320 Free PMC article.
-
uPAR Knockout Results in a Deep Glycolytic and OXPHOS Reprogramming in Melanoma and Colon Carcinoma Cell Lines.Cells. 2020 Jan 28;9(2):308. doi: 10.3390/cells9020308. Cells. 2020. PMID: 32012858 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

