A human ErbB2-specific T-cell receptor confers potent antitumor effector functions in genetically engineered primary cytotoxic lymphocytes
- PMID: 25003657
- PMCID: PMC4137348
- DOI: 10.1089/hum.2014.006
A human ErbB2-specific T-cell receptor confers potent antitumor effector functions in genetically engineered primary cytotoxic lymphocytes
Abstract
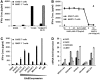
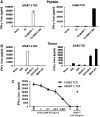
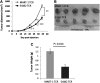
The ErbB2 protein is a member of the tyrosine kinase family of growth factor receptors that is overexpressed in cancers of the breast, ovary, stomach, kidney, colon, and lung, and therefore represents an attractive candidate antigen for targeted cancer immunotherapy. Cytotoxic T lymphocytes specific for various immunogenic ErbB2 peptides have been described, but they often exhibit both poor functional avidity and tumor reactivity. In order to generate potent CD8(+) T cells with specificity for the ErbB2(369-377) peptide, we performed one round of in vitro peptide stimulation of CD8(+) T cells isolated from an HLA-A2(+) patient who was previously vaccinated with autologous dendritic cells pulsed with HLA class I ErbB2 peptides. Using this approach, we enriched highly avid ErbB2-reactive T cells with strong ErbB2-specific, antitumor effector functions. We then stimulated these ErbB2-reactive T cells with ErbB2(+) HLA-A2(+) tumor cells in vitro and sorted tumor-activated ErbB2(369-377) peptide T cells, which allowed for the isolation of a novel T-cell receptor (TCR) with ErbB2(369-377) peptide specificity. Primary human CD8(+) T cells genetically modified to express this ErbB2-specific TCR specifically bound ErbB2(369-377) peptide containing HLA-A2 tetramers, and efficiently recognized target cells pulsed with low nanomolar concentrations of ErbB2(369-377) peptide as well as nonpulsed ErbB2(+) HLA-A2(+) tumor cell lines in vitro. In a novel xenograft model, ErbB2-redirected T cells also significantly delayed progression of ErbB2(+) HLA-A2(+) human tumor in vivo. Together, these results support the notion that redirection of normal T-cell specificity by TCR gene transfer can have potential applications in the adoptive immunotherapy of ErbB2-expressing malignancies.
Figures


References
-
- Ahmadi M., King J.W., Xue S.A., et al. (2011). CD3 limits the efficacy of TCR gene therapy in vivo. Blood 118, 3528–3537 - PubMed
-
- Anderson B.W., Peoples G.E., Murray J.L., et al. (2000). Peptide priming of cytolytic activity to HER-2 epitope 369–377 in healthy individuals. Clin. Cancer Res. 6, 4192–4200 - PubMed
-
- Bartsch R., Wenzel C., Zielinski C.C., and Steger G.G. (2007). HER-2-positive breast cancer: hope beyond trastuzumab. BioDrugs 21, 69–77 - PubMed
-
- Bookman M.A., Darcy K.M., Clarke-Pearson D., et al. (2003). Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 21, 283–290 - PubMed
-
- Brossart P., Stuhler G., Flad T., et al. (1998). Her-2/neu-derived peptides are tumor-associated antigens expressed by human renal cell and colon carcinoma lines and are recognized by in vitro induced specific cytotoxic T lymphocytes. Cancer Res. 58, 732–736 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

