Unraveling the secrets of white matter--bridging the gap between cellular, animal and human imaging studies
- PMID: 25003711
- PMCID: PMC4155933
- DOI: 10.1016/j.neuroscience.2014.06.058
Unraveling the secrets of white matter--bridging the gap between cellular, animal and human imaging studies
Abstract
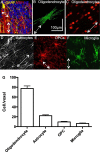
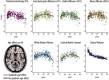
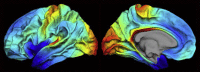
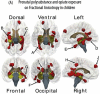
The CNS white matter makes up about half of the human brain, and with advances in human imaging it is increasingly becoming clear that changes in the white matter play a major role in shaping human behavior and learning. However, the mechanisms underlying these white matter changes remain poorly understood. Within this special issue of Neuroscience on white matter, recent advances in our knowledge of the function of white matter, from the molecular level to human imaging, are reviewed. Collaboration between fields is essential to understand the function of the white matter, but due to differences in methods and field-specific 'language', communication is often hindered. In this review, we try to address this hindrance by introducing the methods and providing a basic background to myelin biology and human imaging as a prelude to the other reviews within this special issue.
Keywords: DTI; anatomy; functional imaging; glial cell; myelin; white matter.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures




Similar articles
-
White Matter Plasticity in the Adult Brain.Neuron. 2017 Dec 20;96(6):1239-1251. doi: 10.1016/j.neuron.2017.11.026. Neuron. 2017. PMID: 29268094 Free PMC article. Review.
-
Transmission Electron Microscopy and Morphometry of the CNS White Matter.Methods Mol Biol. 2020;2143:233-261. doi: 10.1007/978-1-0716-0585-1_18. Methods Mol Biol. 2020. PMID: 32524485
-
Evaluation of diffusion kurtosis imaging in ex vivo hypomyelinated mouse brains.Neuroimage. 2016 Jan 1;124(Pt A):612-626. doi: 10.1016/j.neuroimage.2015.09.028. Epub 2015 Sep 21. Neuroimage. 2016. PMID: 26400013 Free PMC article.
-
The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants.Neuroscience. 2014 Sep 12;276:48-71. doi: 10.1016/j.neuroscience.2013.12.044. Epub 2013 Dec 28. Neuroscience. 2014. PMID: 24378955 Review.
-
Periods of synchronized myelin changes shape brain function and plasticity.Nat Neurosci. 2021 Nov;24(11):1508-1521. doi: 10.1038/s41593-021-00917-2. Epub 2021 Oct 28. Nat Neurosci. 2021. PMID: 34711959 Review.
Cited by
-
Cardiorespiratory fitness and white matter integrity in Alzheimer's disease.Brain Imaging Behav. 2016 Sep;10(3):660-8. doi: 10.1007/s11682-015-9431-3. Brain Imaging Behav. 2016. PMID: 26239997 Free PMC article.
-
Associations between modifiable risk factors and white matter of the aging brain: insights from diffusion tensor imaging studies.Neurobiol Aging. 2019 Aug;80:56-70. doi: 10.1016/j.neurobiolaging.2019.04.006. Epub 2019 Apr 11. Neurobiol Aging. 2019. PMID: 31103633 Free PMC article. Review.
-
Tidying up white matter: Neuroplastic transformations in sensorimotor tracts following slackline skill acquisition.Hum Brain Mapp. 2024 Nov;45(16):e26791. doi: 10.1002/hbm.26791. Hum Brain Mapp. 2024. PMID: 39524014 Free PMC article. Clinical Trial.
-
Up-Regulation of Oligodendrocyte Lineage Markers in the Cerebellum of Autistic Patients: Evidence from Network Analysis of Gene Expression.Mol Neurobiol. 2016 Aug;53(6):4019-4025. doi: 10.1007/s12035-015-9351-7. Epub 2015 Jul 21. Mol Neurobiol. 2016. PMID: 26189831
-
A macroscopic link between interhemispheric tract myelination and cortico-cortical interactions during action reprogramming.Nat Commun. 2022 Jul 22;13(1):4253. doi: 10.1038/s41467-022-31687-5. Nat Commun. 2022. PMID: 35869067 Free PMC article.
References
-
- Alexander D.C., Hubbard P.L., Hall M.G., Moore E.A., Ptito M., Parker G.J., Dyrby T.B. Orientationally invariant indices of axon diameter and density from diffusion MRI. Neuroimage. 2010;52:1374–1389. - PubMed
-
- Almeida R.G., Lyons D.A. On the resemblance of synapse formation and CNS myelination. Neuroscience. 2014;276:98–108. - PubMed
-
- Amlien I., Fjell A.M. Diffusion tensor imaging of white matter degeneration in Alzheimer’s disease and mild cognitive impairment. Neuroscience. 2014;276:206–215. - PubMed
-
- Basser P.J., Mattiello J., LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

