Snail regulates Nanog status during the epithelial-mesenchymal transition via the Smad1/Akt/GSK3β signaling pathway in non-small-cell lung cancer
- PMID: 25003810
- PMCID: PMC4116528
- DOI: 10.18632/oncotarget.2006
Snail regulates Nanog status during the epithelial-mesenchymal transition via the Smad1/Akt/GSK3β signaling pathway in non-small-cell lung cancer
Abstract
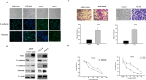
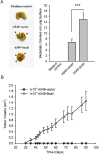
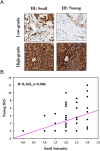
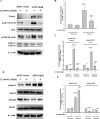
The epithelial-mesenchymal transition (EMT), a crucial step in cancer metastasis, is important in transformed cancer cells with stem cell-like properties. In this study, we established a Snail-overexpressing cell model for non-small-cell lung cancer (NSCLC) and investigated its underlying mechanism. We also identified the downstream molecular signaling pathway that contributes to the role of Snail in regulating Nanog expression. Our data shows that high levels of Snail expression correlate with metastasis and high levels of Nanog expression in NSCLC. NSCLC cells expressing Snail are characterized by active EMT characteristics and exhibit an increased ability to migrate, chemoresistance, sphere formation, and stem cell-like properties. We also investigated the signals required for Snail-mediated Nanog expression. Our data demonstrate that LY294002, SB431542, LDN193189, and Noggin pretreatment inhibit Snail-induced Nanog expression during EMT. This study shows a significant correlation between Snail expression and phosphorylation of Smad1, Akt, and GSK3β. In addition, pretreatment with SB431542, LDN193189, or Noggin prevented Snail-induced Smad1 and Akt hyperactivation and reactivated GSK3β. Moreover, LY294002 pretreatment prevented Akt hyperactivation and reactivated GSK3β without altering Smad1 activation. These findings provide a novel mechanistic insight into the important role of Snail in NSCLC during EMT and indicate potentially useful therapeutic targets for NSCLC.
Figures




Similar articles
-
Coexpression of gene Oct4 and Nanog initiates stem cell characteristics in hepatocellular carcinoma and promotes epithelial-mesenchymal transition through activation of Stat3/Snail signaling.J Hematol Oncol. 2015 Mar 11;8:23. doi: 10.1186/s13045-015-0119-3. J Hematol Oncol. 2015. PMID: 25879771 Free PMC article.
-
Acquisition of epithelial-mesenchymal transition phenotype and cancer stem cell-like properties in cisplatin-resistant lung cancer cells through AKT/β-catenin/Snail signaling pathway.Eur J Pharmacol. 2014 Jan 15;723:156-66. doi: 10.1016/j.ejphar.2013.12.004. Epub 2013 Dec 12. Eur J Pharmacol. 2014. PMID: 24333218
-
The CNPY2 enhances epithelial-mesenchymal transition via activating the AKT/GSK3β pathway in non-small cell lung cancer.Cell Biol Int. 2018 Aug;42(8):959-964. doi: 10.1002/cbin.10961. Epub 2018 Apr 17. Cell Biol Int. 2018. PMID: 29569784
-
Akt2 mediates TGF-β1-induced epithelial to mesenchymal transition by deactivating GSK3β/snail signaling pathway in renal tubular epithelial cells.Cell Physiol Biochem. 2014;34(2):368-82. doi: 10.1159/000363006. Epub 2014 Jul 11. Cell Physiol Biochem. 2014. PMID: 25059120
-
Snail nuclear transport: the gateways regulating epithelial-to-mesenchymal transition?Semin Cancer Biol. 2014 Aug;27:39-45. doi: 10.1016/j.semcancer.2014.06.003. Epub 2014 Jun 17. Semin Cancer Biol. 2014. PMID: 24954011 Free PMC article. Review.
Cited by
-
Decidual stromal cells-derived exosomes incurred insufficient migration and invasion of trophoblast by disturbing of β-TrCP-mediated snail ubiquitination and degradation in unexplained recurrent spontaneous abortion.Eur J Med Res. 2024 Jan 9;29(1):39. doi: 10.1186/s40001-023-01598-2. Eur J Med Res. 2024. PMID: 38195659 Free PMC article.
-
MicroRNA-1305 Inhibits the Stemness of LCSCs and Tumorigenesis by Repressing the UBE2T-Dependent Akt-Signaling Pathway.Mol Ther Nucleic Acids. 2019 Jun 7;16:721-732. doi: 10.1016/j.omtn.2019.04.013. Epub 2019 Apr 22. Mol Ther Nucleic Acids. 2019. PMID: 31128423 Free PMC article.
-
Twist1-related miR-26b-5p suppresses epithelial-mesenchymal transition, migration and invasion by targeting SMAD1 in hepatocellular carcinoma.Oncotarget. 2016 Apr 26;7(17):24383-401. doi: 10.18632/oncotarget.8328. Oncotarget. 2016. PMID: 27027434 Free PMC article.
-
Nanog maintains stemness of Lkb1-deficient lung adenocarcinoma and prevents gastric differentiation.EMBO Mol Med. 2021 Mar 5;13(3):e12627. doi: 10.15252/emmm.202012627. Epub 2021 Jan 13. EMBO Mol Med. 2021. PMID: 33439550 Free PMC article.
-
Isolation and Characterization of Human Colon Adenocarcinoma Stem-Like Cells Based on the Endogenous Expression of the Stem Markers.Int J Mol Sci. 2021 Apr 28;22(9):4682. doi: 10.3390/ijms22094682. Int J Mol Sci. 2021. PMID: 33925224 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. - PubMed
-
- Thiery J. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. - PubMed
-
- Clarke M, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124(6):1111–1115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

