Granger causality revisited
- PMID: 25003817
- PMCID: PMC4176655
- DOI: 10.1016/j.neuroimage.2014.06.062
Granger causality revisited
Abstract
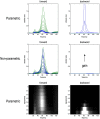
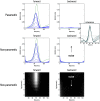
This technical paper offers a critical re-evaluation of (spectral) Granger causality measures in the analysis of biological timeseries. Using realistic (neural mass) models of coupled neuronal dynamics, we evaluate the robustness of parametric and nonparametric Granger causality. Starting from a broad class of generative (state-space) models of neuronal dynamics, we show how their Volterra kernels prescribe the second-order statistics of their response to random fluctuations; characterised in terms of cross-spectral density, cross-covariance, autoregressive coefficients and directed transfer functions. These quantities in turn specify Granger causality - providing a direct (analytic) link between the parameters of a generative model and the expected Granger causality. We use this link to show that Granger causality measures based upon autoregressive models can become unreliable when the underlying dynamics is dominated by slow (unstable) modes - as quantified by the principal Lyapunov exponent. However, nonparametric measures based on causal spectral factors are robust to dynamical instability. We then demonstrate how both parametric and nonparametric spectral causality measures can become unreliable in the presence of measurement noise. Finally, we show that this problem can be finessed by deriving spectral causality measures from Volterra kernels, estimated using dynamic causal modelling.
Keywords: Cross spectra; Dynamic causal modelling; Dynamics; Effective connectivity; Functional connectivity; Granger causality; Neurophysiology.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Barnett L., Seth A. The MVGC multivariate Granger causality toolbox: a new approach to Granger-causal inference. J. Neurosci. Methods. 2014;223:50–68. - PubMed
-
- Barnett L., Barrett A., Seth A. Granger causality and transfer entropy are equivalent for Gaussian variables. Phys. Rev. Lett. 2009;103(23):238701. - PubMed
-
- Boly M., Garrido M.I., Gosseries O., Bruno M.A., Boveroux P., Schnakers C., Massimini M., Litvak V., Laureys S., Friston K. Preserved feedforward but impaired top–down processes in the vegetative state. Science. 2011;332(6031):858–862. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

