miR-196b-mediated translation regulation of mouse insulin2 via the 5'UTR
- PMID: 25003985
- PMCID: PMC4086887
- DOI: 10.1371/journal.pone.0101084
miR-196b-mediated translation regulation of mouse insulin2 via the 5'UTR
Abstract
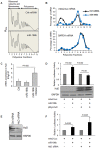
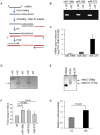
The 5' and the 3' untranslated regions (UTR) of the insulin genes are very well conserved across species. Although microRNAs (miRNAs) are known to regulate insulin secretion process, direct regulation of insulin biosynthesis by miRNA has not been reported. Here, we show that mouse microRNA miR-196b can specifically target the 5'UTR of the long insulin2 splice isoform. Using reporter assays we show that miR-196b specifically increases the translation of the reporter protein luciferase. We further show that this translation activation is abolished when Argonaute 2 levels are knocked down after transfection with an Argonaute 2-directed siRNA. Binding of miR-196b to the target sequence in insulin 5'UTR causes the removal of HuD (a 5'UTR-associated translation inhibitor), suggesting that both miR-196b and HuD bind to the same RNA element. We present data suggesting that the RNA-binding protein HuD, which represses insulin translation, is displaced by miR-196b. Together, our findings identify a mechanism of post-transcriptional regulation of insulin biosynthesis.
Conflict of interest statement
Figures




References
-
- Wessells NK, Evans J (1968) Ultrastructural studies of early morphogenesis and cytodifferentiation in the embryonic mammalian pancreas. Dev Biol 17(4): 413–46. - PubMed
-
- Docherty K, Clark AR (1994) Nutrient regulation of insulin gene expression. FASEB J 8(1): 20–7. - PubMed
-
- Itoh N, Sei T, Nose K, Hiroshi O (1978) Glucose stimulation of the proinsulin synthesis in isolated pancreatic islets without increasing amount of proinsulin mRNA. FEBS Lett 93(2): 343–7. - PubMed
-
- Wicksteed B, Herbert TP, Alarcon C, Lingohr MK, Moss LG, et al. (2001) Cooperativity between the preproinsulin mRNA untranslated regions is necessary for glucose-stimulated translation. J Biol Chem 276(25): 22553–8. - PubMed
-
- Muralidharan B, Bakthavachalu B, Pathak A, Seshadri V (2007) A minimal element in 5'UTR of insulin mRNA mediates its translational regulation by glucose. FEBS Lett 581(21): 4103–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

