IL-10 and lymphotoxin-α expression profiles within marginal zone-like B-cell populations are associated with control of HIV-1 disease progression
- PMID: 25003989
- PMCID: PMC4087016
- DOI: 10.1371/journal.pone.0101949
IL-10 and lymphotoxin-α expression profiles within marginal zone-like B-cell populations are associated with control of HIV-1 disease progression
Abstract
Understanding how the immune system facilitates or controls HIV-1 disease progression has important implications for the design of effective interventions. We report that although B-cell dysregulations associated with HIV-1 disease progression are accompanied by an overall decrease in the percentage of total blood B-cells, we observe an increase in relative frequencies of cells presenting characteristics of both transitional immature and first-line marginal zone (MZ) B-cell populations, we designated as precursor MZ-like B-cells. B-cells with similar attributes have been associated with IL-10 expression and "regulatory" potential. As such, the relative frequencies of precursor MZ-like B-cells expressing IL-10 are increased in the blood of viremic HIV-1-infected individuals when compared to HIV-negative subjects. Importantly, in aviremic HIV-1 Elite-Controllers (EC), we found unaltered relative percentages of precursor MZ-like B-cells which presented normal IL-10 expression patterns. Furthermore, EC had increased relative frequencies of blood MZ-like B-cells expressing LT-α. Thus in contrast to viremic HIV-1-infected individuals, EC present MZ-like B-cell populations which IL-10 and LT-α expression profiles may favour homeostasis of immune responses and lymphoid microenvironments.
Conflict of interest statement
Figures
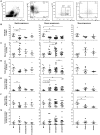
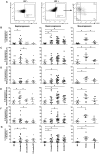
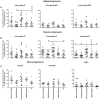
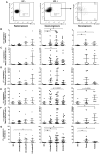
References
-
- Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, et al. (1998) Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95: 163–175. - PubMed
-
- Poudrier J, Weng X, Kay DG, Paré G, Calvo EL, et al. (2001) The AIDS disease of CD4C/HIV transgenic mice shows impaired germinal centers and autoantibodies and develops in the absence of IFN-gamma and IL-6. Immunity 15: 173–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

