Combined effects of ultrasound and immobilization protocol on butyl acetate synthesis catalyzed by CALB
- PMID: 25004067
- PMCID: PMC6271129
- DOI: 10.3390/molecules19079562
Combined effects of ultrasound and immobilization protocol on butyl acetate synthesis catalyzed by CALB
Abstract
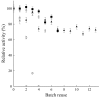
It is well established that the performance of lipase B from Candida antarctica (CALB) as catalyst for esterification reactions may be improved by the use of ultrasound technology or by its immobilization on styrene-divinylbenzene beads (MCI-CALB). The present research evaluated the synthesis of butyl acetate using MCI-CALB under ultrasonic energy, comparing the results against those obtained using the commercial preparation, Novozym 435. The optimal conditions were determined using response surface methodology (RSM) evaluating the following parameters: reaction temperature, substrate molar ratio, amount of biocatalyst, and added water. The optimal conditions for butyl acetate synthesis catalyzed by MCI-CALB were: temperature, 48.8 °C; substrate molar ratio, 3.46:1 alcohol:acid; amount of biocatalyst, 7.5%; and added water 0.28%, both as substrate mass. Under these conditions, 90% of conversion was reached in 1.5 h. In terms of operational stability, MCI-CALB was reused in seven cycles while keeping 70% of its initial activity under ultrasonic energy. The support pore size and resistance are key points for the enzyme activity and stability under mechanical stirring. The use of ultrasound improved both activity and stability because of better homogeneity and reduced mechanical stress to the immobilized system.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



References
-
- Yahya A.R.M., Anderson W.A., Moo-Young M. Ester synthesis in lipase-catalyzed reactions. Enzyme Microb. Technol. 1998;23:438–450. doi: 10.1016/S0141-0229(98)00065-9. - DOI
-
- Milašinović N., Knežević-Jugović Z., Jakovljević Ž., Filipović J., Kalagasidis Krušić M. Synthesis of n-amyl isobutyrate catalyzed by Candida rugosa lipase immobilized into poly(N-isopropylacrylamide-co-itaconic acid) hydrogels. Chem. Eng. J. 2012;181–182:614–623. doi: 10.1016/j.cej.2011.11.115. - DOI
-
- Friedrich J.L.R., Peña F.P., Garcia-Galan C., Fernandez-Lafuente R., Ayub M.A.Z., Rodrigues R.C. Effect of immobilization protocol on optimal conditions of ethyl butyrate synthesis catalyzed by lipase B from Candida antarctica. J. Chem. Technol. Biotechnol. 2013;88:1089–1095. doi: 10.1002/jctb.3945. - DOI
-
- Lozano P., Bernal J.M., Navarro A. A clean enzymatic process for producing flavour esters by direct esterification in switchable ionic liquid/solid phases. Green Chem. 2012;14:3026–3033. doi: 10.1039/c2gc36081k. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

