Ionic liquid-based vacuum microwave-assisted extraction followed by macroporous resin enrichment for the separation of the three glycosides salicin, hyperin and rutin from Populus bark
- PMID: 25004075
- PMCID: PMC6271344
- DOI: 10.3390/molecules19079689
Ionic liquid-based vacuum microwave-assisted extraction followed by macroporous resin enrichment for the separation of the three glycosides salicin, hyperin and rutin from Populus bark
Abstract
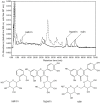
An effective ionic liquid vacuum microwave-assisted method was developed for extraction of the thermo- and oxygen-sensitive glycosides salicin, hyperin and rutin from Populus bark due to the strong solvating effects of ionic liquids on plant cell walls. In this study, [C4mim]BF4 solution was selected as the extracting solution for extraction of the target analytes. After optimization by single factor experiments and response surface methodology, the optimum condition parameters were achieved, which included 1.0 M [C4mim]BF4, 2 h soaking time, -0.08 MPa vacuum, 20 min microwave irradiation time, 400 W microwave irradiation power and 25 mL/g liquid/solid ratio. Under the optimum conditions, higher extraction yields of salicin (35.53 mg/g), hyperin (1.32 mg/g) and rutin (2.40 mg/g) were obtained. Compared with other extraction methods, the developed method provided higher yields of the three target components after a relatively shorter extraction time (20 min). No obvious degradation of the target analytes was observed under the optimum conditions in performed stability studies and the proposed method had a high reproducibility. Meanwhile, after adsorption and desorption on macroporous D101 resin, the target analytes can be effectively separated from the [C4mim]BF4 ionic liquid extraction solution and the yields of salicin, hyperin and rutin were 89%, 82% and 84%, respectively. The recovered [C4mim]BF4 ionic liquid presented a good extraction effect on the three analytes after recycling five times.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures




Similar articles
-
Application of ionic liquids in vacuum microwave-assisted extraction followed by macroporous resin isolation of three flavonoids rutin, hyperoside and hesperidin from Sorbus tianschanica leaves.J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Mar 1;1014:45-55. doi: 10.1016/j.jchromb.2016.01.045. Epub 2016 Feb 2. J Chromatogr B Analyt Technol Biomed Life Sci. 2016. PMID: 26874229
-
Optimization of ionic liquid based simultaneous ultrasonic- and microwave-assisted extraction of rutin and quercetin from leaves of velvetleaf (Abutilon theophrasti) by response surface methodology.ScientificWorldJournal. 2014;2014:283024. doi: 10.1155/2014/283024. Epub 2014 Aug 27. ScientificWorldJournal. 2014. PMID: 25243207 Free PMC article.
-
Development of an ionic liquid-based microwave-assisted method for the extraction and determination of taxifolin in different parts of Larix gmelinii.Molecules. 2014 Nov 25;19(12):19471-90. doi: 10.3390/molecules191219471. Molecules. 2014. PMID: 25429567 Free PMC article.
-
Ionic liquid-based microwave-assisted extraction of rutin from Chinese medicinal plants.Talanta. 2010 Dec 15;83(2):582-90. doi: 10.1016/j.talanta.2010.10.006. Epub 2010 Oct 8. Talanta. 2010. PMID: 21111178
-
Ionic liquid-based microwave-assisted extraction for the determination of flavonoid glycosides in pigeon pea leaves by high-performance liquid chromatography-diode array detector with pentafluorophenyl column.J Sep Sci. 2012 Nov;35(21):2875-83. doi: 10.1002/jssc.201200473. Epub 2012 Sep 24. J Sep Sci. 2012. PMID: 23001940
Cited by
-
Antitumor activity of Polygonatum sibiricum polysaccharides.Arch Pharm Res. 2024 Sep;47(8-9):696-708. doi: 10.1007/s12272-024-01511-3. Epub 2024 Jul 26. Arch Pharm Res. 2024. PMID: 39060656 Review.
-
An Efficient Ionic Liquid-Mediated Extraction and Enrichment of Isoimperatorin from Ostericum koreanum (Max.) Kitagawa.Molecules. 2021 Oct 29;26(21):6555. doi: 10.3390/molecules26216555. Molecules. 2021. PMID: 34770966 Free PMC article.
-
Enrichment and Purification of Aucubin from Eucommia ulmoides Ionic Liquid Extract Using Macroporous Resins.Materials (Basel). 2018 Sep 18;11(9):1758. doi: 10.3390/ma11091758. Materials (Basel). 2018. PMID: 30231478 Free PMC article.
-
Microwave-assisted simultaneous extraction of luteolin and apigenin from tree peony pod and evaluation of its antioxidant activity.ScientificWorldJournal. 2014;2014:506971. doi: 10.1155/2014/506971. Epub 2014 Oct 22. ScientificWorldJournal. 2014. PMID: 25405227 Free PMC article.
-
Special Effect of Ionic Liquids on the Extraction of Flavonoid Glycosides from Chrysanthemum morifolium Ramat by Microwave Assistance.Molecules. 2015 Apr 28;20(5):7683-99. doi: 10.3390/molecules20057683. Molecules. 2015. PMID: 25927899 Free PMC article.
References
-
- Wang X., Wang Q., Xu G.J., Xu L. Advances in the research of constituents and pharmacology of Populus L. Nat. Prod. Res. Develop. 1999;11:65–74.
-
- Zhang C., Zheng H., Liu G., Hu F. Development and validation of HPLC method for determination of salicin in poplar buds: Application for screening of counterfeit propolis. Food Chem. 2011;127:345–350.
-
- Li M.M., Liu A.T., Zou C.J., Xu W.D., Shimizu H., Wang K.Y. An overview of the “Three-North” Shelterbelt project in China. For. Stud. China. 2012;14:70–79.
-
- Clausen T.P., Reichardt P.B., Bryant J.P., Werner R.A., Post K., Frisby K. Chemical model for short-term induction in quaking aspen (Populus tremuloides) foliage against herbivores. J. Chem. Ecol. 1989;15:2335–2346. - PubMed
-
- Nan Y., Zhou L. Comparative study: Salicin and rutin contents in propolis, and poplar leaves and buds. World Sci. Tech-Moder. Tradit. Chin. Med. Mater. Med. 2008;10:59–61.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

