Cell fusion enhances mesendodermal differentiation of human induced pluripotent stem cells
- PMID: 25004077
- PMCID: PMC4235980
- DOI: 10.1089/scd.2014.0120
Cell fusion enhances mesendodermal differentiation of human induced pluripotent stem cells
Abstract
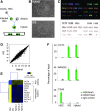
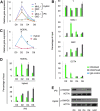
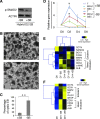
Human induced pluripotent stem cells (iPS cells) resemble embryonic stem cells and can differentiate into cell derivatives of all three germ layers. However, frequently the differentiation efficiency of iPS cells into some lineages is rather poor. Here, we found that fusion of iPS cells with human hematopoietic stem cells (HSCs) enhances iPS cell differentiation. Such iPS hybrids showed a prominent differentiation bias toward hematopoietic lineages but also toward other mesendodermal lineages. Additionally, during differentiation of iPS hybrids, expression of early mesendodermal markers-Brachyury (T), MIX1 Homeobox-Like Protein 1 (MIXL1), and Goosecoid (GSC)-appeared with faster kinetics than in parental iPS cells. Following iPS hybrid differentiation there was a prominent induction of NODAL and inhibition of NODAL signaling blunted mesendodermal differentiation. This indicates that NODAL signaling is critically involved in mesendodermal bias of iPS hybrid differentiation. In summary, we demonstrate that iPS cell fusion with HSCs prominently enhances iPS cell differentiation.
Figures

Similar articles
-
Mesodermal and hematopoietic differentiation from ES and iPS cells.Stem Cell Rev Rep. 2013 Aug;9(4):422-34. doi: 10.1007/s12015-012-9388-1. Stem Cell Rev Rep. 2013. PMID: 22684542 Review.
-
Induced pluripotent stem cells expressing elevated levels of sox-2, oct-4, and klf-4 are severely reduced in their differentiation from mesodermal to hematopoietic progenitor cells.Stem Cells Dev. 2011 Jul;20(7):1131-42. doi: 10.1089/scd.2010.0391. Epub 2011 Feb 24. Stem Cells Dev. 2011. PMID: 21348597
-
The balance of positive and negative effects of TGF-β signaling regulates the development of hematopoietic and endothelial progenitors in human pluripotent stem cells.Stem Cells Dev. 2013 Oct 15;22(20):2765-76. doi: 10.1089/scd.2013.0008. Epub 2013 Jul 19. Stem Cells Dev. 2013. PMID: 23758278 Free PMC article.
-
SMYD2 Drives Mesendodermal Differentiation of Human Embryonic Stem Cells Through Mediating the Transcriptional Activation of Key Mesendodermal Genes.Stem Cells. 2019 Nov;37(11):1401-1415. doi: 10.1002/stem.3068. Epub 2019 Aug 12. Stem Cells. 2019. PMID: 31348575
-
Hematopoietic cell differentiation from embryonic and induced pluripotent stem cells.Stem Cell Res Ther. 2013 Jun 18;4(3):71. doi: 10.1186/scrt222. Stem Cell Res Ther. 2013. PMID: 23796405 Free PMC article. Review.
Cited by
-
Differentiation-Defective Human Induced Pluripotent Stem Cells Reveal Strengths and Limitations of the Teratoma Assay and In Vitro Pluripotency Assays.Stem Cell Reports. 2017 May 9;8(5):1340-1353. doi: 10.1016/j.stemcr.2017.03.009. Stem Cell Reports. 2017. PMID: 28494940 Free PMC article.
-
The difficulty to model Huntington's disease in vitro using striatal medium spiny neurons differentiated from human induced pluripotent stem cells.Sci Rep. 2021 Mar 25;11(1):6934. doi: 10.1038/s41598-021-85656-x. Sci Rep. 2021. PMID: 33767215 Free PMC article.
-
Cell Cluster Sorting in Automated Differentiation of Patient-specific Induced Pluripotent Stem Cells Towards Blood Cells.Front Bioeng Biotechnol. 2022 May 12;10:755983. doi: 10.3389/fbioe.2022.755983. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35662848 Free PMC article.
-
Role of Hox genes in stem cell differentiation.World J Stem Cells. 2015 Apr 26;7(3):583-95. doi: 10.4252/wjsc.v7.i3.583. World J Stem Cells. 2015. PMID: 25914765 Free PMC article. Review.
-
The role of Nav1.7 in human nociceptors: insights from human induced pluripotent stem cell-derived sensory neurons of erythromelalgia patients.Pain. 2019 Jun;160(6):1327-1341. doi: 10.1097/j.pain.0000000000001511. Pain. 2019. PMID: 30720580 Free PMC article.
References
-
- Takahashi K. and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 - PubMed
-
- Bellin M, Marchetto MC, Gage FH. and Mummery CL. (2012). Induced pluripotent stem cells: the new patient?. Nat Rev Mol Cell Biol 13:713–726 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
