Mitophagy switches cell death from apoptosis to necrosis in NSCLC cells treated with oncolytic measles virus
- PMID: 25004098
- PMCID: PMC4116530
- DOI: 10.18632/oncotarget.2028
Mitophagy switches cell death from apoptosis to necrosis in NSCLC cells treated with oncolytic measles virus
Abstract
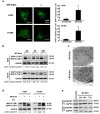
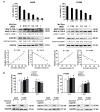
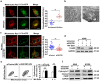
Although apoptotic phenomena have been observed in malignant cells infected by measles virus vaccine strain Edmonston B (MV-Edm), the precise oncolytic mechanisms are poorly defined. In this study we found that MV-Edm induced autophagy and sequestosome 1-mediated mitophagy leading to decreased cytochrome c release, which blocked the pro-apoptotic cascade in non-small cell lung cancer cells (NSCLCs). The decrease of apoptosis by mitophagy favored viral replication. Persistent viral replication sustained by autophagy ultimately resulted in necrotic cell death due to ATP depletion. Importantly, when autophagy was impaired in NSCLCs MV-Edm-induced cell death was significantly abrogated despite of increased apoptosis. Taken together, our results define a novel oncolytic mechanism by which mitophagy switches cell death from apoptosis to more efficient necrosis in NSCLCs following MV-Edm infection. This provides a foundation for future improvement of oncolytic virotherapy or antiviral therapy.
Conflict of interest statement
None of the authors have a financial interest to declare.
Figures


Similar articles
-
Mitophagy enhances oncolytic measles virus replication by mitigating DDX58/RIG-I-like receptor signaling.J Virol. 2014 May;88(9):5152-64. doi: 10.1128/JVI.03851-13. Epub 2014 Feb 26. J Virol. 2014. PMID: 24574393 Free PMC article.
-
[Synergistic Effect of NF-κB Signaling Pathway Inhibitor and Oncolytic Measles Virus Vaccine Strain against Lung Cancer and Underlying Mechanisms].Zhongguo Fei Ai Za Zhi. 2021 Apr 20;24(4):245-253. doi: 10.3779/j.issn.1009-3419.2021.102.14. Epub 2021 Mar 29. Zhongguo Fei Ai Za Zhi. 2021. PMID: 33775041 Free PMC article. Chinese.
-
Engineered measles virus as a novel oncolytic therapy against prostate cancer.Prostate. 2009 Jan 1;69(1):82-91. doi: 10.1002/pros.20857. Prostate. 2009. PMID: 18973133 Free PMC article.
-
Attenuated oncolytic measles virus strains as cancer therapeutics.Curr Pharm Biotechnol. 2012 Jul;13(9):1732-41. doi: 10.2174/138920112800958896. Curr Pharm Biotechnol. 2012. PMID: 21740361 Free PMC article. Review.
-
Measles to the Rescue: A Review of Oncolytic Measles Virus.Viruses. 2016 Oct 22;8(10):294. doi: 10.3390/v8100294. Viruses. 2016. PMID: 27782084 Free PMC article. Review.
Cited by
-
Investigating the interplay between mitophagy and diabetic neuropathy: Uncovering the hidden secrets of the disease pathology.Pharmacol Res. 2024 Oct;208:107394. doi: 10.1016/j.phrs.2024.107394. Epub 2024 Sep 3. Pharmacol Res. 2024. PMID: 39233055 Review.
-
The essential role of mitochondrial dynamics in antiviral immunity.Mitochondrion. 2018 Jul;41:21-27. doi: 10.1016/j.mito.2017.11.007. Epub 2017 Dec 5. Mitochondrion. 2018. PMID: 29246869 Free PMC article. Review.
-
Viral strategies for triggering and manipulating mitophagy.Autophagy. 2018;14(10):1665-1673. doi: 10.1080/15548627.2018.1466014. Epub 2018 Aug 16. Autophagy. 2018. PMID: 29895192 Free PMC article.
-
The Role of Mitophagy in Regulating Cell Death.Oxid Med Cell Longev. 2021 May 18;2021:6617256. doi: 10.1155/2021/6617256. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34113420 Free PMC article. Review.
-
Viral pathogen-induced mechanisms to antagonize mammalian interferon (IFN) signaling pathway.Cell Mol Life Sci. 2021 Feb;78(4):1423-1444. doi: 10.1007/s00018-020-03671-z. Epub 2020 Oct 21. Cell Mol Life Sci. 2021. PMID: 33084946 Free PMC article. Review.
References
-
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16(1):3–11. - PMC - PubMed
-
- Duprez L, Wirawan E, Vanden Berghe T, Vandenabeele P. Major cell death pathways at a glance. Microbes Infect. 2009;11(13):1050–1062. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

