Prodrug applications for targeted cancer therapy
- PMID: 25004822
- PMCID: PMC4147050
- DOI: 10.1208/s12248-014-9638-z
Prodrug applications for targeted cancer therapy
Abstract
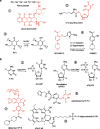
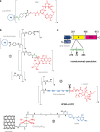
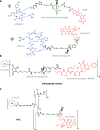
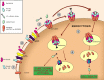
Prodrugs are widely used in the targeted delivery of cytotoxic compounds to cancer cells. To date, targeted prodrugs for cancer therapy have achieved great diversity in terms of target selection, activation chemistry, as well as size and physicochemical nature of the prodrug. Macromolecular prodrugs such as antibody-drug conjugates, targeted polymer-drug conjugates and other conjugates that self-assemble to form liposomal and micellar nanoparticles currently represent a major trend in prodrug development for cancer therapy. In this review, we explore a unified view of cancer-targeted prodrugs and highlight several examples from recombinant technology that exemplify the prodrug concept but are not identified as such. Recombinant "prodrugs" such as engineered anthrax toxin show promise in biological specificity through the conditionally targeting of multiple cellular markers. Conditional targeting is achieved by structural complementation, the spontaneous assembly of engineered inactive subunits or fragments to reconstitute functional activity. These complementing systems can be readily adapted to achieve conditionally bispecific targeting of enzymes that are used to activate low-molecular weight prodrugs. By leveraging strengths from medicinal chemistry, polymer science, and recombinant technology, prodrugs are poised to remain a core component of highly focused and tailored strategies aimed at conditionally attacking complex molecular phenotypes in clinically relevant cancer.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

