Sample size calculations in pediatric clinical trials conducted in an ICU: a systematic review
- PMID: 25004909
- PMCID: PMC4107993
- DOI: 10.1186/1745-6215-15-274
Sample size calculations in pediatric clinical trials conducted in an ICU: a systematic review
Abstract
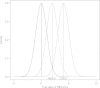
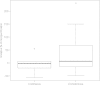
At the design stage of a clinical trial, several assumptions have to be made. These usually include guesses about parameters that are not of direct interest but must be accounted for in the analysis of the treatment effect and also in the sample size calculation (nuisance parameters, e.g. the standard deviation or the control group event rate). We conducted a systematic review to investigate the impact of misspecification of nuisance parameters in pediatric randomized controlled trials conducted in intensive care units. We searched MEDLINE through PubMed. We included all publications concerning two-arm RCTs where efficacy assessment was the main objective. We included trials with pharmacological interventions. Only trials with a dichotomous or a continuous outcome were included. This led to the inclusion of 70 articles describing 71 trials. In 49 trial reports a sample size calculation was reported. Relative misspecification could be calculated for 28 trials, 22 with a dichotomous and 6 with a continuous primary outcome. The median [inter-quartile range (IQR)] overestimation was 6.9 [-12.1, 57.8]% for the control group event rate in trials with dichotomous outcomes and -1.5 [-15.3, 5.1]% for the standard deviation in trials with continuous outcomes. Our results show that there is room for improvement in the clear reporting of sample size calculations in pediatric clinical trials conducted in ICUs. Researchers should be aware of the importance of nuisance parameters in study design and in the interpretation of the results.
Figures



References
-
- Noordzij M, Tripepi G, Dekker FW, Zoccali C, Tanck MW, Jager KJ. Sample size calculations: basic principles and common pitfalls. Nephrol Dial Transplant. 2010;25:1388–1393. - PubMed
-
- Schulz KF, Grimes DA. Sample size calculations in randomised trials: mandatory and mystical. Lancet. 2005;365:1348–1353. - PubMed
-
- Eng J. Sample size estimation: how many individuals should be studied? Radiology. 2003;227:309–313. - PubMed
-
- Halpern SD, Karlawish JH, Berlin JA. The continuing unethical conduct of underpowered clinical trials. JAMA. 2002;288:358–362. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

