The effects of propofol cardioplegia on blood and myocardial biomarkers of stress and injury in patients with isolated coronary artery bypass grafting or aortic valve replacement using cardiopulmonary bypass: protocol for a single-center randomized controlled trial
- PMID: 25004932
- PMCID: PMC4115261
- DOI: 10.2196/resprot.3353
The effects of propofol cardioplegia on blood and myocardial biomarkers of stress and injury in patients with isolated coronary artery bypass grafting or aortic valve replacement using cardiopulmonary bypass: protocol for a single-center randomized controlled trial
Abstract
Background: Despite improved myocardial protection strategies, cardioplegic arrest and ischemia still result in reperfusion injury. We have previously published a study describing the effects of propofol (an anesthetic agent commonly used in cardiac surgery) on metabolic stress, cardiac function, and injury in a clinically relevant animal model. We concluded that cardioplegia supplementation with propofol at a concentration relevant to the human clinical setting resulted in improved hemodynamic function, reduced oxidative stress, and reduced reperfusion injury when compared to standard cardioplegia.
Objective: The Propofol cardioplegia for Myocardial Protection Trial (ProMPT) aims to translate the successful animal intervention to the human clinical setting. We aim to test the hypothesis that supplementation of the cardioplegic solution with propofol will be cardioprotective for patients undergoing isolated coronary artery bypass graft or aortic valve replacement surgery with cardiopulmonary bypass.
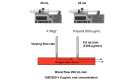
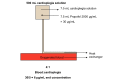
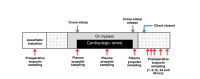
Methods: The trial is a single-center, placebo-controlled, randomized trial with blinding of participants, health care staff, and the research team. Patients aged between 18 and 80 years undergoing nonemergency isolated coronary artery bypass graft or aortic valve replacement surgery with cardiopulmonary bypass at the Bristol Heart Institute are being invited to participate. Participants are randomly assigned in a 1:1 ratio to either cardioplegia supplementation with propofol (intervention) or cardioplegia supplementation with intralipid (placebo) using a secure, concealed, Internet-based randomization system. Randomization is stratified by operation type and minimized by diabetes mellitus status. Biomarkers of cardiac injury and metabolism are being assessed to investigate any cardioprotection conferred. The primary outcome is myocardial injury, studied by measuring myocardial troponin T. The trial is designed to test hypotheses about the superiority of the intervention within each surgical stratum. The sample size of 96 participants has been chosen to achieve 80% power to detect standardized differences of 0.5 at a significance level of 5% (2-tailed) assuming equal numbers in each surgical stratum.
Results: A total of 96 patients have been successfully recruited over a 2-year period. Results are to be published in late 2014.
Conclusions: Designing a practicable method for delivering a potentially protective dose of propofol to the heart during cardiac surgery was challenging. If our approach confirms the potential of propofol to reduce damage during cardiac surgery, we plan to design a larger multicenter trial to detect differences in clinical outcomes.
Trial registration: International Standard Randomized Controlled Trial Number (ISRCTN): 84968882; http://www.controlled-trials.com/ISRCTN84968882/ProMPT (Archived by WebCite at http://www.webcitation.org/6Qi8A51BS).
Keywords: anesthetics; aortic valve; cardiac surgery; cardioplegia; cardiopulmonary bypass; clinical trials, randomized; coronary artery; ischemia; reperfusion; troponin.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
Efficacy of propofol-supplemented cardioplegia on biomarkers of organ injury in patients having cardiac surgery using cardiopulmonary bypass: A protocol for a randomised controlled study (ProMPT2).Perfusion. 2024 May;39(4):722-732. doi: 10.1177/02676591231157269. Epub 2023 Feb 16. Perfusion. 2024. PMID: 36794486 Free PMC article.
-
Propofol cardioplegia: A single-center, placebo-controlled, randomized controlled trial.J Thorac Cardiovasc Surg. 2015 Dec;150(6):1610-9.e13. doi: 10.1016/j.jtcvs.2015.06.044. Epub 2015 Jun 30. J Thorac Cardiovasc Surg. 2015. PMID: 26256300 Clinical Trial.
-
Efficacy of propofol-supplemented cardioplegia on biomarkers of organ injury in patients having cardiac surgery using cardiopulmonary bypass: a statistical analysis plan for the ProMPT-2 randomised controlled trial.Trials. 2024 Feb 29;25(1):153. doi: 10.1186/s13063-024-08016-w. Trials. 2024. PMID: 38424570 Free PMC article.
-
L-arginine impact on inflammatory and cardiac markers in patients undergoing coronary artery bypass graft: a systematic review and meta-analysis of randomized controlled trials.BMC Cardiovasc Disord. 2024 Nov 13;24(1):641. doi: 10.1186/s12872-024-04318-8. BMC Cardiovasc Disord. 2024. PMID: 39538180 Free PMC article.
-
Cardioprotection in cardiovascular surgery.Basic Res Cardiol. 2024 Aug;119(4):545-568. doi: 10.1007/s00395-024-01062-0. Epub 2024 Jun 10. Basic Res Cardiol. 2024. PMID: 38856733 Review.
Cited by
-
Anesthetic Propofol-Induced Gene Expression Changes in Patients Undergoing Coronary Artery Bypass Graft Surgery Based on Dynamical Differential Coexpression Network Analysis.Comput Math Methods Med. 2016;2016:7097612. doi: 10.1155/2016/7097612. Epub 2016 Jun 29. Comput Math Methods Med. 2016. PMID: 27437027 Free PMC article.
-
Cardioprotection during Adult and Pediatric Open Heart Surgery.Biomed Res Int. 2015;2015:712721. doi: 10.1155/2015/712721. Epub 2015 Jun 23. Biomed Res Int. 2015. PMID: 26161409 Free PMC article. No abstract available.
-
Cardiopulmonary bypass and oxidative stress.Oxid Med Cell Longev. 2015;2015:189863. doi: 10.1155/2015/189863. Epub 2015 Feb 4. Oxid Med Cell Longev. 2015. PMID: 25722792 Free PMC article. Review.
-
Efficacy of propofol-supplemented cardioplegia on biomarkers of organ injury in patients having cardiac surgery using cardiopulmonary bypass: A protocol for a randomised controlled study (ProMPT2).Perfusion. 2024 May;39(4):722-732. doi: 10.1177/02676591231157269. Epub 2023 Feb 16. Perfusion. 2024. PMID: 36794486 Free PMC article.
References
-
- Suleiman MS, Halestrap AP, Griffiths EJ. Mitochondria: a target for myocardial protection. Pharmacol Ther. 2001 Jan;89(1):29–46. - PubMed
-
- Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta. 2009 Nov;1787(11):1402–15. doi: 10.1016/j.bbabio.2008.12.017. http://linkinghub.elsevier.com/retrieve/pii/S0005-2728(09)00007-3 - DOI - PubMed
-
- Forde RC, Fitzgerald DJ. Reactive oxygen species and platelet activation in reperfusion injury. Circulation. 1997 Feb 18;95(4):787–9. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=9054729 - PubMed
-
- Halestrap AP, Kerr PM, Javadov S, Woodfield KY. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta. 1998 Aug 10;1366(1-2):79–94. - PubMed
-
- Ascione R, Caputo M, Gomes WJ, Lotto AA, Bryan AJ, Angelini GD, Suleiman MS. Myocardial injury in hypertrophic hearts of patients undergoing aortic valve surgery using cold or warm blood cardioplegia. Eur J Cardiothorac Surg. 2002 Mar;21(3):440–6. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

