Crystallization screening: the influence of history on current practice
- PMID: 25005076
- PMCID: PMC4089519
- DOI: 10.1107/S2053230X1401262X
Crystallization screening: the influence of history on current practice
Abstract
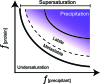
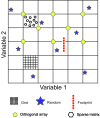
While crystallization historically predates crystallography, it is a critical step for the crystallographic process. The rich history of crystallization and how that history influences current practices is described. The tremendous impact of crystallization screens on the field is discussed.
Keywords: crystallization screening.
Figures



Similar articles
-
Gently does it.Science. 2014 Mar 7;343(6175):1094-7. doi: 10.1126/science.343.6175.1094. Science. 2014. PMID: 24604192 No abstract available.
-
The China connection: Michael Rossmann and his first encounter with me.Protein Cell. 2010 Jan;1(1):6-8. doi: 10.1007/s13238-010-0001-6. Protein Cell. 2010. PMID: 21203992 Free PMC article. No abstract available.
-
Dazzling history.Science. 2014 Mar 7;343(6175):1092-3. doi: 10.1126/science.343.6175.1092. Science. 2014. PMID: 24604191 No abstract available.
-
Protein Crystallization.Methods Mol Biol. 2017;1607:17-50. doi: 10.1007/978-1-4939-7000-1_2. Methods Mol Biol. 2017. PMID: 28573568 Review.
-
Practical macromolecular cryocrystallography.Acta Crystallogr F Struct Biol Commun. 2015 Jun;71(Pt 6):622-42. doi: 10.1107/S2053230X15008304. Epub 2015 May 27. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 26057787 Free PMC article. Review.
Cited by
-
X-Ray Crystallographic Studies for Revealing Binding Sites of General Anesthetics in Pentameric Ligand-Gated Ion Channels.Methods Enzymol. 2018;603:21-47. doi: 10.1016/bs.mie.2018.01.017. Epub 2018 Mar 24. Methods Enzymol. 2018. PMID: 29673527 Free PMC article.
-
A crystallization apparatus for temperature-controlled flow-cell dialysis with real-time visualization.J Appl Crystallogr. 2016 Apr 22;49(Pt 3):806-813. doi: 10.1107/S1600576716004635. eCollection 2016 Jun 1. J Appl Crystallogr. 2016. PMID: 27275137 Free PMC article.
-
Design of Experiments As a Tool for Optimization in Recombinant Protein Biotechnology: From Constructs to Crystals.Mol Biotechnol. 2019 Dec;61(12):873-891. doi: 10.1007/s12033-019-00218-x. Mol Biotechnol. 2019. PMID: 31664704 Review.
-
The MORPHEUS II protein crystallization screen.Acta Crystallogr F Struct Biol Commun. 2015 Jul;71(Pt 7):831-7. doi: 10.1107/S2053230X1500967X. Epub 2015 Jun 27. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 26144227 Free PMC article.
-
A simple technique to reduce evaporation of crystallization droplets by using plate lids with apertures for adding liquids.Acta Crystallogr F Struct Biol Commun. 2014 Dec 1;70(Pt 12):1707-13. doi: 10.1107/S2053230X14025126. Epub 2014 Nov 28. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 25484231 Free PMC article.
References
-
- Asherie, N. (2004). Protein crystallization and phase diagrams. Methods, 34, 266–272. - PubMed
-
- Asherie, N., Ginsberg, C., Blass, S., Greenbaum, A. & Knafo, S. (2008). Solubility of thaumatin. Cryst. Growth Des. 8, 1815–1817.
-
- Ataka, M., Shinzawa-Itoh, K. & Yoshikawa, S. (1992). Phase diagrams of a crystalline membrane protein, bovine heart cytochrome c oxidase, in the salting-in region. J. Cryst. Growth, 122, 60–65.
-
- Ataka, M. & Tanaka, S. (1986). The growth of large single crystals of lysozyme. Biopolymers, 25, 337–350. - PubMed
-
- Atha, D. H. & Ingham, K. C. (1981). Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J. Biol. Chem. 256, 12108–12117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

