Cloning, expression, purification, crystallization and preliminary X-ray diffraction analysis of glyceraldehyde-3-phosphate dehydrogenase from Streptococcus agalactiae NEM316
- PMID: 25005093
- PMCID: PMC4089536
- DOI: 10.1107/S2053230X14011418
Cloning, expression, purification, crystallization and preliminary X-ray diffraction analysis of glyceraldehyde-3-phosphate dehydrogenase from Streptococcus agalactiae NEM316
Abstract
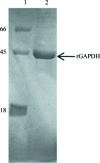
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is an essential enzyme involved in glycolysis. Despite lacking the secretory signal sequence, this cytosolic enzyme has been found localized at the surface of several bacteria and fungi. As a surface protein, GAPDH exhibits various adhesive functions, thereby facilitating colonization and invasion of host tissues. Streptococcus agalactiae, also known as group B streptococcus (GBS), binds onto the host using its surface adhesins and causes sepsis and pneumonia in neonates. GAPDH is one of the surface adhesins of GBS binding to human plasminogen and is a virulent factor associated with host colonization. Although the surface-associated GAPDH has been shown to bind to a variety of host extracellular matrix (ECM) molecules in various bacteria, the molecular mechanism underlying their interaction is not fully understood. To investigate this, structural studies on GAPDH of S. agalactiae were initiated. The gapC gene of S. agalactiae NEM316 encoding GAPDH protein was cloned into pET-28a vector, overexpressed in Escherichia coli BL21(DE3) cells and purified to homogeneity. The purified protein was crystallized using the hanging-drop vapour-diffusion method. The GAPDH crystals obtained in two different crystallization conditions diffracted to 2.8 and 2.6 Å resolution, belonging to two different space groups P2₁ and P2₁2₁2₁, respectively. The structure was solved by molecular replacement and structure refinement is now in progress.
Keywords: glyceraldehyde-3-phosphate dehydrogenase; group B streptococcus; moonlighting; plasminogen; surface adhesin.
Figures

Similar articles
-
Structure of Streptococcus agalactiae glyceraldehyde-3-phosphate dehydrogenase holoenzyme reveals a novel surface.Acta Crystallogr F Struct Biol Commun. 2014 Oct;70(Pt 10):1333-9. doi: 10.1107/S2053230X14019517. Epub 2014 Sep 25. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 25286935 Free PMC article.
-
Crystal structure of GAPDH of Streptococcus agalactiae and characterization of its interaction with extracellular matrix molecules.Microb Pathog. 2019 Feb;127:359-367. doi: 10.1016/j.micpath.2018.12.020. Epub 2018 Dec 12. Microb Pathog. 2019. PMID: 30553015
-
High-resolution crystal structure of Streptococcus agalactiae glyceraldehyde-3-phosphate dehydrogenase.Acta Crystallogr F Struct Biol Commun. 2018 Apr 1;74(Pt 4):236-244. doi: 10.1107/S2053230X18003801. Epub 2018 Mar 23. Acta Crystallogr F Struct Biol Commun. 2018. PMID: 29633972 Free PMC article.
-
Expression, purification, enzymatic characterization and crystallization of glyceraldehyde-3-phosphate dehydrogenase from Naegleria gruberi, the first one from phylum Percolozoa.Protein Expr Purif. 2016 Nov;127:125-130. doi: 10.1016/j.pep.2016.07.007. Epub 2016 Jul 15. Protein Expr Purif. 2016. PMID: 27426132 Review.
-
Anchorless Bacterial Moonlighting Metabolic Enzymes Modulate the Immune System and Contribute to Pathogenesis.ACS Infect Dis. 2024 Aug 9;10(8):2551-2566. doi: 10.1021/acsinfecdis.4c00323. Epub 2024 Jul 27. ACS Infect Dis. 2024. PMID: 39066728 Review.
Cited by
-
Structure of Streptococcus agalactiae glyceraldehyde-3-phosphate dehydrogenase holoenzyme reveals a novel surface.Acta Crystallogr F Struct Biol Commun. 2014 Oct;70(Pt 10):1333-9. doi: 10.1107/S2053230X14019517. Epub 2014 Sep 25. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 25286935 Free PMC article.
References
-
- Arisoy, A. S., Altinişik, B., Tünger, O., Kurutepe, S. & Ispahi, C. (2003). Infection, 31, 244–246. - PubMed
-
- Dumke, R., Hausner, M. & Jacobs, E. (2011). Microbiology, 157, 2328–2338. - PubMed
-
- Ferreira-da-Silva, F., Pereira, P. J., Gales, L., Roessle, M., Svergun, D. I., Moradas-Ferreira, P. & Damas, A. M. (2006). J. Biol. Chem. 281, 33433–33440. - PubMed
-
- Fröhlicher, S., Reichen-Fahrni, G., Müller, M., Surbek, D., Droz, S., Spellerberg, B. & Sendi, P. (2014). Swiss Med. Wkly, 144, w13935. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

