Recent advances in live cell imaging of hepatoma cells
- PMID: 25005127
- PMCID: PMC4108253
- DOI: 10.1186/1471-2121-15-26
Recent advances in live cell imaging of hepatoma cells
Abstract
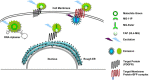
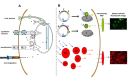
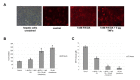
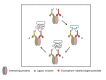
Live cell imaging enables the study of dynamic processes of living cells in real time by use of suitable reporter proteins and the staining of specific cellular structures and/or organelles. With the availability of advanced optical devices and improved cell culture protocols it has become a rapidly growing research methodology. The success of this technique relies mainly on the selection of suitable reporter proteins, construction of recombinant plasmids possessing cell type specific promoters as well as reliable methods of gene transfer. This review aims to provide an overview of the recent developments in the field of marker proteins (bioluminescence and fluorescent) and methodologies (fluorescent resonance energy transfer, fluorescent recovery after photobleaching and proximity ligation assay) employed as to achieve an improved imaging of biological processes in hepatoma cells. Moreover, different expression systems of marker proteins and the modes of gene transfer are discussed with emphasis on the study of lipid droplet formation in hepatocytes as an example.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

