Endothelial dysfunction: the link between homocysteine and hydrogen sulfide
- PMID: 25005183
- PMCID: PMC5539954
- DOI: 10.2174/0929867321666140706142335
Endothelial dysfunction: the link between homocysteine and hydrogen sulfide
Abstract
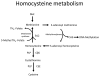
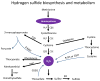
High level of homocysteine (hyperhomocysteinemia, HHcy) is associated with increased risk for vascular disease. Evidence for this emerges from epidemiological studies which show that HHcy is associated with premature peripheral, coronary artery and cerebrovascular disease independent of other risk factors. Possible mechanisms by which homocysteine causes vascular injury include endothelial injury, DNA dysfunction, proliferation of smooth muscle cells, increased oxidative stress, reduced activity of glutathione peroxidase and promoting inflammation. HHcy has been shown to cause direct damage to endothelial cells both in vitro and in vivo. Clinically, this manifests as impaired flow-mediated vasodilation and is mainly due to a reduction in nitric oxide synthesis and bioavailability. The effect of impaired nitric oxide release can in turn trigger and potentiate atherothrombogenesis and oxidative stress. Endothelial damage is a crucial aspect of atherosclerosis and precedes overt manifestation of disease. In addition, endothelial dysfunction is also associated with hypertension, diabetes, ischemia reperfusion injury and neurodegenerative diseases. Homocysteine is a precursor of hydrogen sulfide (H2S) which is formed by transulfuration process catalyzed by the enzymes, cystathionine β-synthase and cystathionine γ-lyase. H2S is a gasotransmitter that has emerged recently as a novel mediator in cardiovascular homeostasis. As a potent vasodilator, it plays several roles which include regulation of vessel diameter, protection of endothelium from redox stress, ischemia reperfusion injury and chronic inflammation. However, the precise mechanism by which it mediates these beneficial effects is complex and still remains unclear. Current evidence indicates H2S modulates cellular functions by a variety of intracellular signaling processes. In this review, we summarize the mechanisms of HHcy-induced endothelial dysfunction and the metabolism and physiological functions of H2S as a protective agent.
Conflict of interest statement
Figures
References
-
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. - PubMed
-
- Dusting GJ, Moncada S, Mullane KM, Vane JR. Implications of prostacyclin generation for modulation of vascular tone. Clinical science and molecular medicine Supplement. 1978;4:195s–198s. - PubMed
-
- Moncada S, Higgs EA, Vane JR. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977;1(8001):18–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

