IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia
- PMID: 25005243
- PMCID: PMC5189717
- DOI: 10.1038/leu.2014.215
IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia
Abstract
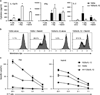
Disease relapse or progression is a major cause of death following umbilical cord blood (UCB) transplantation (UCBT) in patients with high-risk, relapsed or refractory acute lymphoblastic leukemia (ALL). Adoptive transfer of donor-derived T cells modified to express a tumor-targeted chimeric antigen receptor (CAR) may eradicate persistent disease after transplantation. Such therapy has not been available to UCBT recipients, however, due to the low numbers of available UCB T cells and the limited capacity for ex vivo expansion of cytolytic cells. We have developed a novel strategy to expand UCB T cells to clinically relevant numbers in the context of exogenous cytokines. UCB-derived T cells cultured with interleukin (IL)-12 and IL-15 generated >150-fold expansion with a unique central memory/effector phenotype. Moreover, UCB T cells were modified to both express the CD19-specific CAR, 1928z, and secrete IL-12. 1928z/IL-12 UCB T cells retained a central memory-effector phenotype and had increased antitumor efficacy in vitro. Furthermore, adoptive transfer of 1928z/IL-12 UCB T cells resulted in significantly enhanced survival of CD19(+) tumor-bearing SCID-Beige mice. Clinical translation of CAR-modified UCB T cells could augment the graft-versus-leukemia effect after UCBT and thus further improve disease-free survival of transplant patients with B-cell ALL.
Conflict of interest statement
The remaining authors declare no conflict of interest.
Figures





References
-
- Ponce DM, Zheng J, Gonzales AM, Lubin M, Heller G, Castro-Malaspina H, et al. Reduced late mortality risk contributes to similar survival after double-unit cord blood transplantation compared with related and unrelated donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1316–1326. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

