Harnessing developmental processes for vascular engineering and regeneration
- PMID: 25005471
- PMCID: PMC4197623
- DOI: 10.1242/dev.102194
Harnessing developmental processes for vascular engineering and regeneration
Abstract
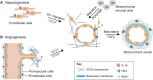
The formation of vasculature is essential for tissue maintenance and regeneration. During development, the vasculature forms via the dual processes of vasculogenesis and angiogenesis, and is regulated at multiple levels: from transcriptional hierarchies and protein interactions to inputs from the extracellular environment. Understanding how vascular formation is coordinated in vivo can offer valuable insights into engineering approaches for therapeutic vascularization and angiogenesis, whether by creating new vasculature in vitro or by stimulating neovascularization in vivo. In this Review, we will discuss how the process of vascular development can be used to guide approaches to engineering vasculature. Specifically, we will focus on some of the recently reported approaches to stimulate therapeutic angiogenesis by recreating the embryonic vascular microenvironment using biomaterials for vascular engineering and regeneration.
Keywords: Angiogenesis; Biomaterials; Stem cells; Tissue engineering; Vasculogenesis.
© 2014. Published by The Company of Biologists Ltd.
Figures

References
-
- Baranski J. D., Chaturvedi R. R., Stevens K. R., Eyckmans J., Carvalho B., Solorzano R. D., Yang M. T., Miller J. S., Bhatia S. N., Chen C. S. (2013). Geometric control of vascular networks to enhance engineered tissue integration and function. Proc. Natl. Acad. Sci. U.S.A. 110, 7586-7591. 10.1073/pnas.1217796110 - DOI - PMC - PubMed
-
- Bloom D. E., Cafiero E. T., Jané-Llopis E., Abrahams-Gessel S., Bloom L. R., Fathima S., Feigl A. B., Gaziano T., Mowafi M., Pandya A., et al. (2011). The Global Economic Burden of Noncommunicable Diseases. Geneva: World Economic Forum
-
- Brudno Y., Ennett-Shepard A. B., Chen R. R., Aizenberg M., Mooney D. J. (2013). Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials 34, 9201-9209. 10.1016/j.biomaterials.2013.08.007 - DOI - PMC - PubMed
-
- Busnadiego O., Gonzalez-Santamaria J., Lagares D., Guinea-Viniegra J., Pichol-Thievend C., Muller L., Rodriguez-Pascual F. (2013). LOXL4 is induced by transforming growth factor beta1 through Smad and JunB/Fra2 and contributes to vascular matrix remodeling. Mol. Cell. Biol. 33, 2388-2401. 10.1128/MCB.00036-13 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

