The BRAzil MAGnesium (BRAMAG) trial: a randomized clinical trial of oral magnesium supplementation in pregnancy for the prevention of preterm birth and perinatal and maternal morbidity
- PMID: 25005784
- PMCID: PMC4096428
- DOI: 10.1186/1471-2393-14-222
The BRAzil MAGnesium (BRAMAG) trial: a randomized clinical trial of oral magnesium supplementation in pregnancy for the prevention of preterm birth and perinatal and maternal morbidity
Abstract
Background: Preterm birth is the leading cause of infant mortality globally, including Brazil. We will evaluate whether oral magnesium citrate reduces the risk of placental dysfunction and its negative consequences for both the fetus and mother, which, in turn, should reduce the need for indicated preterm delivery.
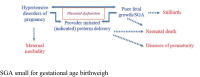
Methods/design: We will complete a multicenter, randomized double-blind clinical trial comparing oral magnesium citrate 150 mg twice daily (n = 2000 women) to matched placebo (n = 1000 women), starting at 121/7 to 206/7 weeks gestation and continued until delivery. We will include women at higher risk for placental dysfunction, based on clinical factors from a prior pregnancy (e.g., prior preterm delivery, stillbirth or preeclampsia) or the current pregnancy (e.g., chronic hypertension, pre-pregnancy diabetes mellitus, maternal age > 35 years or pre-pregnancy maternal body mass index > 30 kg/m2). The primary perinatal outcome is a composite of preterm birth < 37 weeks gestation, stillbirth > 20 weeks gestation, neonatal death < 28 days, or SGA birthweight < 3rd percentile. The primary composite maternal outcome is preeclampsia arising < 37 weeks gestation, severe non-proteinuric hypertension arising < 37 weeks gestation, placental abruption, maternal stroke during pregnancy or ≤ 7 days after delivery, or maternal death during pregnancy or ≤ 7 days after delivery.
Discussion: The results of this randomized clinical trial may be especially relevant in low and middle income countries that have high rates of prematurity and limited resources for acute newborn and maternal care.
Trial registration: ClinicalTrials.gov Identifier NCT02032186, registered December 19, 2013.
Figures
References
-
- Preterm birth. http://www.who.int/mediacentre/factsheets/fs363/en/
-
- Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–535. - PubMed
-
- Simhan HN, Caritis SN. Prevention of preterm delivery. N Engl J Med. 2007;357:477–487. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical