Sawhorse waveform voltammetry for selective detection of adenosine, ATP, and hydrogen peroxide
- PMID: 25005825
- PMCID: PMC4368507
- DOI: 10.1021/ac501229c
Sawhorse waveform voltammetry for selective detection of adenosine, ATP, and hydrogen peroxide
Abstract
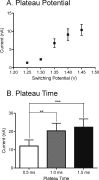
Fast-scan cyclic voltammetry (FSCV) is an electrochemistry technique which allows subsecond detection of neurotransmitters in vivo. Adenosine detection using FSCV has become increasingly popular but can be difficult because of interfering agents which oxidize at or near the same potential as adenosine. Triangle shaped waveforms are traditionally used for FSCV, but modified waveforms have been introduced to maximize analyte sensitivity and provide stability at high scan rates. Here, a modified sawhorse waveform was used to maximize the time for adenosine oxidation and to manipulate the shapes of cyclic voltammograms (CVs) of analytes which oxidize at the switching potential. The optimized waveform consists of scanning at 400 V/s from -0.4 to 1.35 V and holding briefly for 1.0 ms followed by a ramp back down to -0.4 V. This waveform allows the use of a lower switching potential for adenosine detection. Hydrogen peroxide and ATP also oxidize at the switching potential and can interfere with adenosine measurements in vivo; however, their CVs were altered with the sawhorse waveform and they could be distinguished from adenosine. Principal component analysis (PCA) was used to determine that the sawhorse waveform was better than the triangle waveform at discriminating between adenosine, hydrogen peroxide, and ATP. In slices, mechanically evoked adenosine was identified with PCA and changes in the ratio of ATP to adenosine were observed after manipulation of ATP metabolism by POM-1. The sawhorse waveform is useful for adenosine, hydrogen peroxide, and ATP discrimination and will facilitate more confident measurements of these analytes in vivo.
Figures




Similar articles
-
Drift Subtraction for Fast-Scan Cyclic Voltammetry Using Double-Waveform Partial-Least-Squares Regression.Anal Chem. 2019 Jun 4;91(11):7319-7327. doi: 10.1021/acs.analchem.9b01083. Epub 2019 May 23. Anal Chem. 2019. PMID: 31081629 Free PMC article.
-
Voltammetric detection of Neuropeptide Y using a modified sawhorse waveform.Anal Bioanal Chem. 2024 Sep;416(21):4807-4818. doi: 10.1007/s00216-024-05373-y. Epub 2024 Jun 25. Anal Bioanal Chem. 2024. PMID: 38914733 Free PMC article.
-
Scalene Waveform for Codetection of Guanosine and Adenosine Using Fast-Scan Cyclic Voltammetry.Anal Chem. 2019 May 7;91(9):5987-5993. doi: 10.1021/acs.analchem.9b00450. Epub 2019 Apr 15. Anal Chem. 2019. PMID: 30938508
-
Recent advances in fast-scan cyclic voltammetry.Analyst. 2020 Feb 17;145(4):1087-1102. doi: 10.1039/c9an01925a. Analyst. 2020. PMID: 31922162 Free PMC article. Review.
-
Fundamentals of fast-scan cyclic voltammetry for dopamine detection.Analyst. 2020 Feb 17;145(4):1158-1168. doi: 10.1039/c9an01586h. Analyst. 2020. PMID: 31922176 Free PMC article. Review.
Cited by
-
In Vitro Biofouling Performance of Boron-Doped Diamond Microelectrodes for Serotonin Detection Using Fast-Scan Cyclic Voltammetry.Biosensors (Basel). 2023 May 25;13(6):576. doi: 10.3390/bios13060576. Biosensors (Basel). 2023. PMID: 37366941 Free PMC article.
-
Tracking Carbon Microelectrode Impedance during Fast-Scan Cyclic Voltammetry.ACS Sens. 2025 May 23;10(5):3617-3627. doi: 10.1021/acssensors.5c00401. Epub 2025 May 9. ACS Sens. 2025. PMID: 40345216
-
Direct, Real-Time Detection of Adenosine Triphosphate Release from Astrocytes in Three-Dimensional Culture Using an Integrated Electrochemical Aptamer-Based Sensor.ACS Chem Neurosci. 2019 Apr 17;10(4):2070-2079. doi: 10.1021/acschemneuro.9b00033. Epub 2019 Feb 20. ACS Chem Neurosci. 2019. PMID: 30754968 Free PMC article.
-
Achieving reproducible performance of electrochemical, folding aptamer-based sensors on microelectrodes: challenges and prospects.Anal Chem. 2014 Nov 18;86(22):11417-24. doi: 10.1021/ac503407e. Epub 2014 Nov 4. Anal Chem. 2014. PMID: 25337781 Free PMC article.
-
Recent Advances in In Vivo Neurochemical Monitoring.Micromachines (Basel). 2021 Feb 18;12(2):208. doi: 10.3390/mi12020208. Micromachines (Basel). 2021. PMID: 33670703 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

