Clinical use and safety of a novel gentamicin-releasing resorbable bone graft substitute in the treatment of osteomyelitis/osteitis
- PMID: 25005841
- PMCID: PMC4112779
- DOI: 10.1302/2046-3758.37.2000301
Clinical use and safety of a novel gentamicin-releasing resorbable bone graft substitute in the treatment of osteomyelitis/osteitis
Abstract
Objective: A clinical investigation into a new bone void filler is giving first data on systemic and local exposure to the anti-infective substance after implantation.
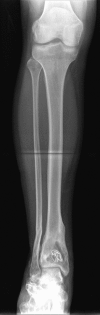
Method: A total of 20 patients with post-traumatic/post-operative bone infections were enrolled in this open-label, prospective study. After radical surgical debridement, the bone cavity was filled with this material. The 21-day hospitalisation phase included determination of gentamicin concentrations in plasma, urine and wound exudate, assessment of wound healing, infection parameters, implant resorption, laboratory parameters, and adverse event monitoring. The follow-up period was six months.
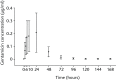
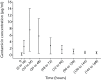
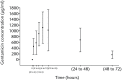
Results: Systemic exposure to gentamicin after implantation was very low as local gentamicin concentrations were measured in wound exudate after six to ten hours. There were no signs of infectious complication throughout the clinical phase. Four patients had recurrent infections several weeks to months after implantation. The outcome was deemed successful by remission of infection in 16 (80%) of these problematic long-term treated patients. Safety laboratory measurements did not indicate nephrotoxic or hepatotoxic effects.
Conclusions: Local application of calcium sulphate/carbonate bone void filler comprising gentamicin revealed sufficient active local levels of the antibiotic by simultaneous significant low systemic exposure in patients with mostly chronic osteomyelitis/osteitis. The material was safe and well tolerated. Cite this article: Bone Joint Res 2014;3:223-9.
Keywords: Calcium sulfate; Gentamicin; Local antibiotic therapy; Osteomyelitis; Resorbable bone void filler.
©2014 The British Editorial Society of Bone & Joint Surgery.
Conflict of interest statement
Figures





Similar articles
-
The levels of vancomycin in the blood and the wound after the local treatment of bone and soft-tissue infection with antibiotic-loaded calcium sulphate as carrier material.Bone Joint J. 2017 Nov;99-B(11):1537-1544. doi: 10.1302/0301-620X.99B11.BJJ-2016-0298.R3. Bone Joint J. 2017. PMID: 29092996
-
Efficacy and Safety of Antibiotic Impregnated Microporous Nanohydroxyapatite Beads for Chronic Osteomyelitis Treatment: A Multicenter, Open-Label, Prospective Cohort Study.Antibiotics (Basel). 2023 Jun 15;12(6):1049. doi: 10.3390/antibiotics12061049. Antibiotics (Basel). 2023. PMID: 37370370 Free PMC article.
-
Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: a prospective series of 100 cases.Bone Joint J. 2016 Sep;98-B(9):1289-96. doi: 10.1302/0301-620X.98B9.38057. Bone Joint J. 2016. PMID: 27587534
-
Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: a review.Injury. 2006 May;37 Suppl 2:S105-12. doi: 10.1016/j.injury.2006.04.016. Injury. 2006. PMID: 16651063 Review.
-
Local application of gentamicin-containing collagen implant in the prophylaxis and treatment of surgical site infection in orthopaedic surgery.Int J Surg. 2012;10 Suppl 1:S15-20. doi: 10.1016/j.ijsu.2012.05.020. Epub 2012 May 30. Int J Surg. 2012. PMID: 22659311 Review.
Cited by
-
Topical Antibiotic Elution in a Collagen-Rich Hydrogel Successfully Inhibits Bacterial Growth and Biofilm Formation In Vitro.Antimicrob Agents Chemother. 2020 Sep 21;64(10):e00136-20. doi: 10.1128/AAC.00136-20. Print 2020 Sep 21. Antimicrob Agents Chemother. 2020. PMID: 32690648 Free PMC article.
-
Clinical experience of debridement combined with resorbable bone graft substitute mixed with antibiotic in the treatment for infants with osteomyelitis.J Orthop Surg Res. 2018 Aug 30;13(1):218. doi: 10.1186/s13018-018-0916-9. J Orthop Surg Res. 2018. PMID: 30165867 Free PMC article.
-
Clinical Application of Antimicrobial Bone Graft Substitute in Osteomyelitis Treatment: A Systematic Review of Different Bone Graft Substitutes Available in Clinical Treatment of Osteomyelitis.Biomed Res Int. 2016;2016:6984656. doi: 10.1155/2016/6984656. Epub 2016 Jan 21. Biomed Res Int. 2016. PMID: 26904683 Free PMC article.
-
Antibiotic Containing Bone Substitute in Major Hip Surgery: A Long Term Gentamicin Elution Study.J Bone Jt Infect. 2018 Apr 12;3(2):68-72. doi: 10.7150/jbji.23901. eCollection 2018. J Bone Jt Infect. 2018. PMID: 29761068 Free PMC article.
-
Novel calcium phosphate cement with biofilm-inhibition and platelet lysate delivery to enhance osteogenesis of encapsulated human periodontal ligament stem cells.Mater Sci Eng C Mater Biol Appl. 2021 Sep;128:112306. doi: 10.1016/j.msec.2021.112306. Epub 2021 Jul 10. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 34474857 Free PMC article.
References
-
- Trampuz A, Zimmerli W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr Infect Dis Rep 2008;10:394–403 - PubMed
-
- Zilberman M, Elsner JJ. Antibiotic-eluting medical devices for various applications. J Control Release 2008;130:202–215 - PubMed
-
- Wahlig H, Dingeldein E, Bergmann R, Reuss K. The release of gentamicin from polymethylmethacrylate beads: an experimental and pharmacokinetic study. J Bone Joint Surg [Br] 1978;60-B:270–275 - PubMed
-
- Henry SL, Galloway KP. Local antibacterial therapy for the management of orthopaedic infections. Pharmacokinetic considerations. Clin Pharmacokinet 1995;29:36–45 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

