The anthocyanin reduced tomato mutant demonstrates the role of flavonols in tomato lateral root and root hair development
- PMID: 25006027
- PMCID: PMC4213093
- DOI: 10.1104/pp.114.240507
The anthocyanin reduced tomato mutant demonstrates the role of flavonols in tomato lateral root and root hair development
Abstract
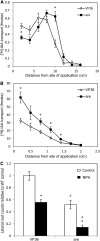
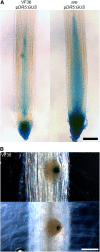
This study utilized tomato (Solanum lycopersicum) mutants with altered flavonoid biosynthesis to understand the impact of these metabolites on root development. The mutant anthocyanin reduced (are) has a mutation in the gene encoding FLAVONOID 3-HYDROXYLASE (F3H), the first step in flavonol synthesis, and accumulates higher concentrations of the F3H substrate, naringenin, and lower levels of the downstream products kaempferol, quercetin, myricetin, and anthocyanins, than the wild type. Complementation of are with the p35S:F3H transgene reduced naringenin and increased flavonols to wild-type levels. The initiation of lateral roots is reduced in are, and p35S:F3H complementation restores wild-type root formation. The flavonoid mutant anthocyanin without has a defect in the gene encoding DIHYDROFLAVONOL REDUCTASE, resulting in elevated flavonols and the absence of anthocyanins and displays increased lateral root formation. These results are consistent with a positive role of flavonols in lateral root formation. The are mutant has increased indole-3-acetic acid transport and greater sensitivity to the inhibitory effect of the auxin transport inhibitor naphthylphthalamic acid on lateral root formation. Expression of the auxin-induced reporter (DR5-β-glucuronidase) is reduced in initiating lateral roots and increased in primary root tips of are. Levels of reactive oxygen species are elevated in are root epidermal tissues and root hairs, and are forms more root hairs, consistent with a role of flavonols as antioxidants that modulate root hair formation. Together, these experiments identify positive roles of flavonols in the formation of lateral roots and negative roles in the formation of root hairs through the modulation of auxin transport and reactive oxygen species, respectively.
© 2014 American Society of Plant Biologists. All Rights Reserved.
Figures





Similar articles
-
Flavonols modulate lateral root emergence by scavenging reactive oxygen species in Arabidopsis thaliana.J Biol Chem. 2021 Jan-Jun;296:100222. doi: 10.1074/jbc.RA120.014543. Epub 2020 Dec 25. J Biol Chem. 2021. PMID: 33839683 Free PMC article.
-
Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato.Plant J. 2010 Jan;61(1):3-15. doi: 10.1111/j.1365-313X.2009.04027.x. Epub 2009 Sep 29. Plant J. 2010. PMID: 19793078
-
Flavonols regulate root hair development by modulating accumulation of reactive oxygen species in the root epidermis.Development. 2020 Apr 27;147(8):dev185819. doi: 10.1242/dev.185819. Development. 2020. PMID: 32179566
-
Flavonols modulate plant development, signaling, and stress responses.Curr Opin Plant Biol. 2023 Apr;72:102350. doi: 10.1016/j.pbi.2023.102350. Epub 2023 Mar 2. Curr Opin Plant Biol. 2023. PMID: 36870100 Free PMC article. Review.
-
Flavonols: old compounds for old roles.Ann Bot. 2011 Nov;108(7):1225-33. doi: 10.1093/aob/mcr234. Epub 2011 Aug 31. Ann Bot. 2011. PMID: 21880658 Free PMC article. Review.
Cited by
-
Hydroxylation decoration patterns of flavonoids in horticultural crops: chemistry, bioactivity and biosynthesis.Hortic Res. 2022 Jan 20;9:uhab068. doi: 10.1093/hr/uhab068. Online ahead of print. Hortic Res. 2022. PMID: 35048127 Free PMC article.
-
Flavonoid deficiency disrupts redox homeostasis and terpenoid biosynthesis in glandular trichomes of tomato.Plant Physiol. 2022 Mar 4;188(3):1450-1468. doi: 10.1093/plphys/kiab488. Plant Physiol. 2022. PMID: 34668550 Free PMC article.
-
Metabolic characteristics of self-pollinated wheat seed under red and blue light during early development.Planta. 2023 Aug 6;258(3):63. doi: 10.1007/s00425-023-04217-w. Planta. 2023. PMID: 37543957
-
The Evolution of Flavonoid Biosynthesis: A Bryophyte Perspective.Front Plant Sci. 2020 Feb 4;11:7. doi: 10.3389/fpls.2020.00007. eCollection 2020. Front Plant Sci. 2020. PMID: 32117358 Free PMC article. Review.
-
Abscisic Acid-Induced Reactive Oxygen Species Are Modulated by Flavonols to Control Stomata Aperture.Plant Physiol. 2017 Dec;175(4):1807-1825. doi: 10.1104/pp.17.01010. Epub 2017 Oct 19. Plant Physiol. 2017. PMID: 29051198 Free PMC article.
References
-
- Agati G, Azzarello E, Pollastri S, Tattini M. (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196: 67–76 - PubMed
-
- Al-Sane K, Povero G, Perata P. (2011) Anthocyanin tomato mutants: overview and characterization of an anthocyanin-less somaclonal mutant. Plant Biosyst 145: 436–444
-
- Azari R, Tadmor Y, Meir A, Reuveni M, Evenor D, Nahon S, Shlomo H, Chen L, Levin I. (2010) Light signaling genes and their manipulation towards modulation of phytonutrient content in tomato fruits. Biotechnol Adv 28: 108–118 - PubMed
-
- Ballester AR, Molthoff J, de Vos R, Hekkert B, Orzaez D, Fernández-Moreno JP, Tripodi P, Grandillo S, Martin C, Heldens J, et al. (2010) Biochemical and molecular analysis of pink tomatoes: deregulated expression of the gene encoding transcription factor SlMYB12 leads to pink tomato fruit color. Plant Physiol 152: 71–84 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

