Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis
- PMID: 25006249
- PMCID: PMC4148880
- DOI: 10.1074/jbc.M114.571620
Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis
Abstract
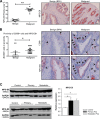
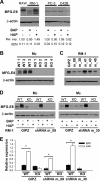
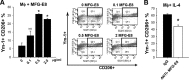
Tumor cells secrete factors that modulate macrophage activation and polarization into M2 type tumor-associated macrophages, which promote tumor growth, progression, and metastasis. The mechanisms that mediate this polarization are not clear. Macrophages are phagocytic cells that participate in the clearance of apoptotic cells, a process known as efferocytosis. Milk fat globule- EGF factor 8 (MFG-E8) is a bridge protein that facilitates efferocytosis and is associated with suppression of proinflammatory responses. This study investigated the hypothesis that MFG-E8-mediated efferocytosis promotes M2 polarization. Tissue and serum exosomes from prostate cancer patients presented higher levels of MFG-E8 compared with controls, a novel finding in human prostate cancer. Coculture of macrophages with apoptotic cancer cells increased efferocytosis, elevated MFG-E8 protein expression levels, and induced macrophage polarization into an alternatively activated M2 phenotype. Administration of antibody against MFG-E8 significantly attenuated the increase in M2 polarization. Inhibition of STAT3 phosphorylation using the inhibitor Stattic decreased efferocytosis and M2 macrophage polarization in vitro, with a correlating increase in SOCS3 protein expression. Moreover, MFG-E8 knockdown tumor cells cultured with wild-type or MFG-E8-deficient macrophages resulted in increased SOCS3 expression with decreased STAT3 activation. This suggests that SOCS3 and phospho-STAT3 act in an inversely dependent manner when stimulated by MFG-E8 and efferocytosis. These results uncover a unique role of efferocytosis via MFG-E8 as a mechanism for macrophage polarization into tumor-promoting M2 cells.
Keywords: Alternative Activation; Bone; Bone Marrow; Efferocytosis; MFG-E8; Macrophage; Phagocytosis; Prostate Cancer; Skeletal Metastasis; Tumor-associated Macrophages.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Bubendorf L., Schöpfer A., Wagner U., Sauter G., Moch H., Willi N., Gasser T. C., Mihatsch M. J. (2000) Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum. Pathol. 31, 578–583 - PubMed
-
- Loberg R. D., Gayed B. A., Olson K. B., Pienta K. J. (2005) A paradigm for the treatment of prostate cancer bone metastases based on an understanding of tumor cell-microenvironment interactions. J. Cell. Biochem. 96, 439–446 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

