A systematic review and mixed-treatment comparison of dapagliflozin with existing anti-diabetes treatments for those with type 2 diabetes mellitus inadequately controlled by sulfonylurea monotherapy
- PMID: 25006351
- PMCID: PMC4085736
- DOI: 10.1186/1758-5996-6-73
A systematic review and mixed-treatment comparison of dapagliflozin with existing anti-diabetes treatments for those with type 2 diabetes mellitus inadequately controlled by sulfonylurea monotherapy
Abstract
Background: To compare the first-in-class sodium glucose co-transporter 2 (SGLT2) inhibitor, dapagliflozin, with existing type 2 diabetes mellitus (T2DM) treatment options available within the European Union (EU) for add-on therapy to sulfonylureas (SUs).
Methods: A systematic review was conducted to identify randomised controlled trials (RCTs) in T2DM patients inadequately controlled by SU monotherapy. Direct meta-analysis, Bucher indirect comparisons and Bayesian network meta-analysis (NMA) were conducted on studies meeting predefined inclusion criteria. Sufficient data were available to assess three clinical endpoints at 24 (+/- 6) weeks follow-up: mean change in HbA1c from baseline, mean change in weight from baseline, and the proportion of patients experiencing at least one episode of hypoglycaemia. The effect of confounding baseline factors was explored through covariate analyses.
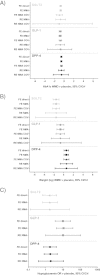
Results: The search identified 1,901 unique citations, with 1,870 excluded based on title/abstract. From reviewing full-texts of the remaining 31 articles, 5 studies were considered eligible for analysis. All studies were comparable in terms of baseline characteristics, including: HbA1c, age and body mass index (BMI). In addition to dapagliflozin, sufficient data for meta-analysis was available for three dipeptidyl peptidase-4 (DPP-4) inhibitors and one glucagon-like peptide-1 (GLP-1) analogue. Based on fixed-effect NMA, all treatment classes resulted in statistically significant decreases in HbA1c at follow-up compared to placebo. Dapagliflozin treatment resulted in significantly decreased weight at follow-up compared to placebo (-1.54 kg; 95% CrI -2.16, -0.92), in contrast to treatment with GLP-1 analogues (-0.65 kg; 95% CrI -1.37, 0.07) and DPP-4 inhibitors (0.57 kg; 95% CrI 0.09, 1.06). The odds of hypoglycaemia were similar to placebo for dapagliflozin and DPP-4 inhibitor add-on treatment, but significantly greater than placebo for GLP-1 analogue add-on treatment (10.89; 95% CrI 4.24, 38.28). Assessment of NMA model heterogeneity was hindered by the small size of the network.
Conclusions: Dapagliflozin, DPP-4 inhibitors and GLP-1 analogues, in combination with SU, all provided better short-term glycaemic control compared to SU monotherapy. Dapagliflozin was the only add-on therapy that had both a favourable weight and hypoglycaemia profile compared to the other classes of treatment evaluated.
Keywords: Dapagliflozin; Diabetes; Mixed treatment comparison; Network meta-analysis; Systematic review.
Figures


References
-
- Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29(7):855–862. - PubMed
-
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–825. - PubMed
-
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

